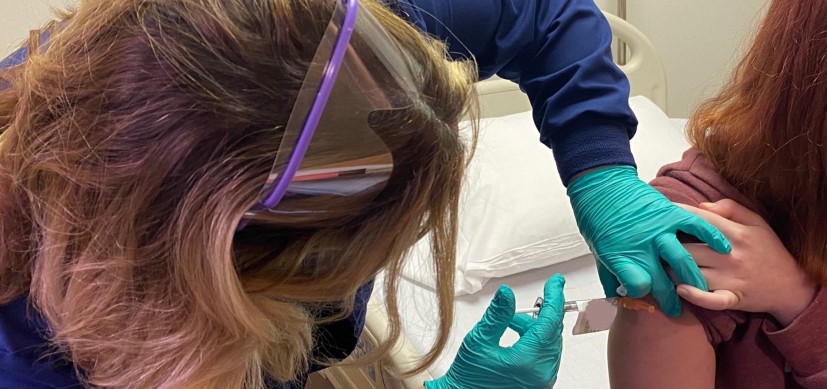
Cincinnati Children’s Plays Central Role in COVID-19 Vaccine Clinical Trials
Post Date: May 22, 2022 | Publish Date:
Dreading the prospect of their children spending weeks in the iron lung or suffering permanent paralysis, parents of the 1950s lined their kids up with urgency to receive polio vaccines. Yet the dangers posed by polio pale in comparison to the COVID-19 pandemic.
The worst polio outbreak in U.S. history occurred in 1952, when a total of 57,628 cases were reported, including 3,145 deaths and 21,269 people struck by varying levels of paralysis. In January 2021 alone, COVID death tolls in America exceeded 3,000 in a single day more than a dozen times, and on just one day of that month new cases exceeded 300,000.
Once again, as the COVID-19 pandemic surged, scientists at Cincinnati Children’s stepped up to the challenge. As a furious global race to develop vaccines produced more than 120 possible candidates, Cincinnati Children’s helped study vaccines made by Moderna, Johnson & Johnson and AztraZenica. It also became one of only four centers to begin evaluating an mRNA vaccine developed by Pfizer and BioNTech, which ultimately became one of the three vaccines approved for use in the United States.
The media blitz that began May 5, 2020—the day the first phase I/II clinical trial of the Pfizer vaccine was announced—continued all the way through 2021. While serving as a leading investigator for one of the most intense clinical trials ever conducted, Robert Frenck, MD, director of the Gamble Vaccine Research Center, also gave hundreds of interviews that were shared worldwide in thousands of news articles translated into numerous languages.

He appeared on CNN, ABC, NBC, CBS, “Good Morning America” and “The Today Show.” He spoke with reporters from the New York Times, Wall Street Journal, Atlantic, Time, Newsweek, STAT, Medscape, Kaiser Health News, and many more. He appeared numerous times on every local news outlet, answering detailed questions about the science behind the vaccines, debunking myths, discussing which activities were safer than others, and constantly repeating the message that no corners were cut despite the speed of the work.
“If you look at a more typical vaccine trial you’d be sending things in batches,” Frenck told CNN. “For this study, they are saying they need information back in 24 hours. We are cutting out the lag time. Because this is an emergency, we are getting people to work as hard as we can.”
In addition to directing the Gamble Center, Frenck is the principal investigator of the NIH-funded Vaccine and Treatment Evaluation Unit (VTEU) at Cincinnati Children’s, one of nine such units nationwide. For years, the VTEU has evaluated annual flu vaccines and has conducted research on a wide range of vaccine candidates for Ebola, MERS, rotavirus, and many other viruses.
As the leader of the pediatric arm, he co-authored Pfizer’s key clinical trial findings, which were published Dec. 10, 2020, in The New England Journal of Medicine. That study became the fourth most-shared paper of more than 2 million studies published in 2020, and the 10th most shared of all papers ever tracked by Altmetric.
The paper was directly cited in more than 2,200 news stories from more than 750 news outlets. It was shared by more than 37,000 Twitter users with a combined 24 million followers. It attracted more than 6,200 readers on Mendeley and has more than 3,400 citations on Dimensions.
The overall Altmetric score for the paper—28796 as of May 16, 2022—set an all-time record for the most-shared paper co-authored by a Cincinnati Children’s faculty member. Its score will continue to climb as more people share or cite the paper in years to come.
Frenck went on to serve as first author in another widely shared paper that focused more specifically on adolescent outcomes of the Pfizer-BioNTech vaccine. This paper, published May 27, 2021, in The New England Journal of Medicine, scored above 7400 on Altmetric and has been shared by Twitter users with nearly 22 million followers.
VACCINE WORK EXTENDED WELL BEYOND PFIZER’S VACCINE
Paul Spearman, MD, director of Infectious Diseases at Cincinnati Children’s, also played a significant national role in COVID-19 vaccine work. He recently completed a four-year term as a member of the Vaccines and Related Biological Products Advisory Committee (VRBPAC), which reviews and evaluates vaccine research and makes recommendations to the FDA.

In October 2020, this committee refused to rush its recommendations despite pressure from the Trump administration to approve emergency use of Pfizer’s vaccine before the November elections. The FDA action occurred in December. Because of Cincinnati Children’s role as a vaccine trial site, Spearman had to recuse himself from some proceedings. Nevertheless, he served as an important media source for explaining how the process works.
Meanwhile, Spearman served as principal investigator for a portion of the clinical trials organized to evaluate Moderna’s COVID vaccine. Adult testing for that vaccine had been overseen locally at the University of Cincinnati. Now, Spearman leads a study of Georgia-based CyanVac’s CVXGA1 vaccine, a nasal spray vaccine candidate for preventing COVID.
FAMILIES STEP UP AMID CONTROVERSY
In August 2020, as political disagreements swirled around vaccination and masking issues, Cincinnati Children’s began a phase III trial of the Pfizer-BioNTech vaccine, including hundreds of adolescents. Oak Hills High School student Katelyn Evans became the first adolescent in the clinical trial.
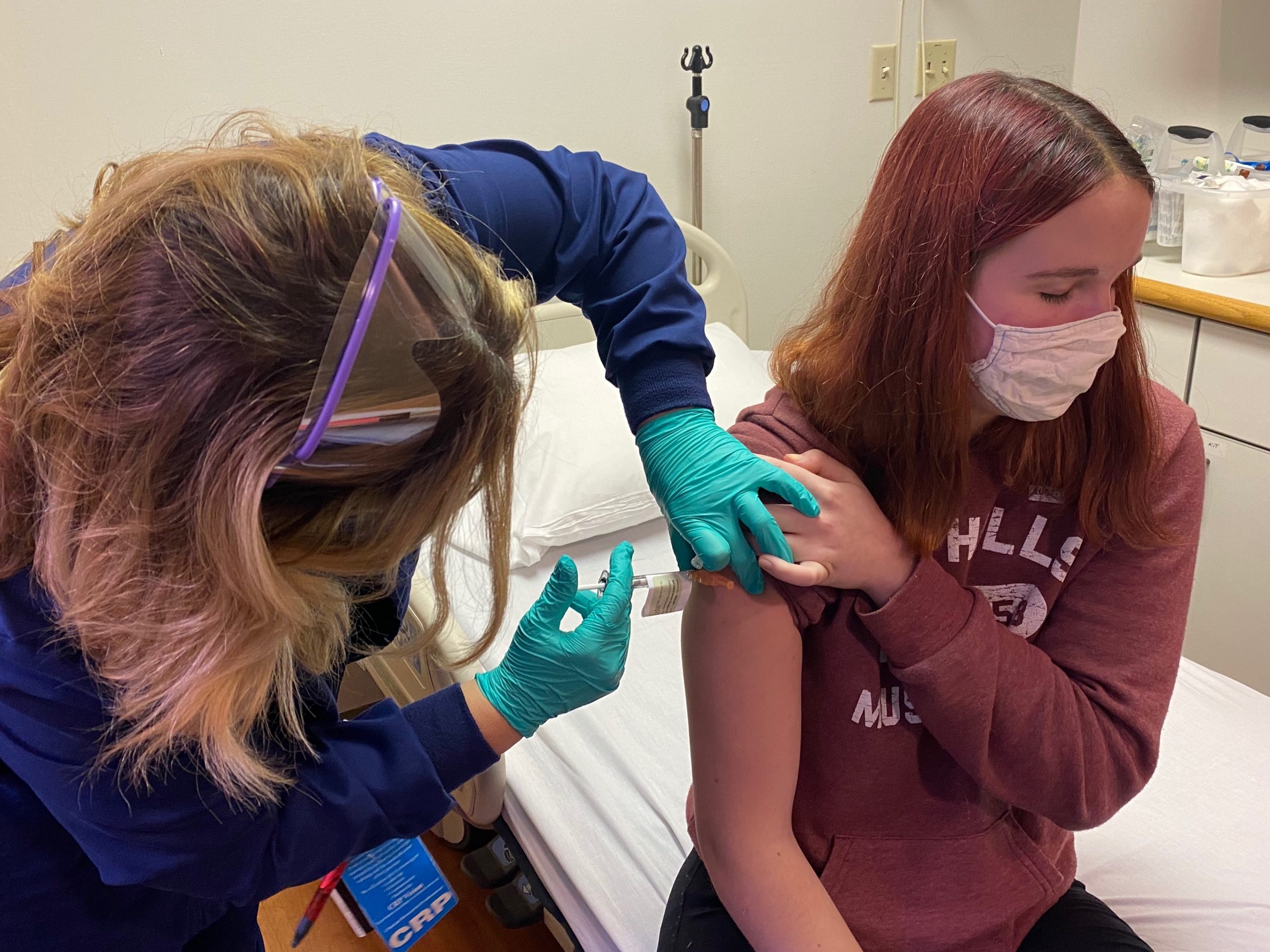
“It’s just really cool knowing that I got to be part of something,” Katelyn told “Good Morning America.”
Meanwhile Melanie Mitchell, 16, a Walnut Hills High School student and daughter of Cincinnati Children’s Monica Mitchell, PhD, told her story to U.S. Sens. Rob Portman and Sherrod Brown in a virtual congressional briefing.
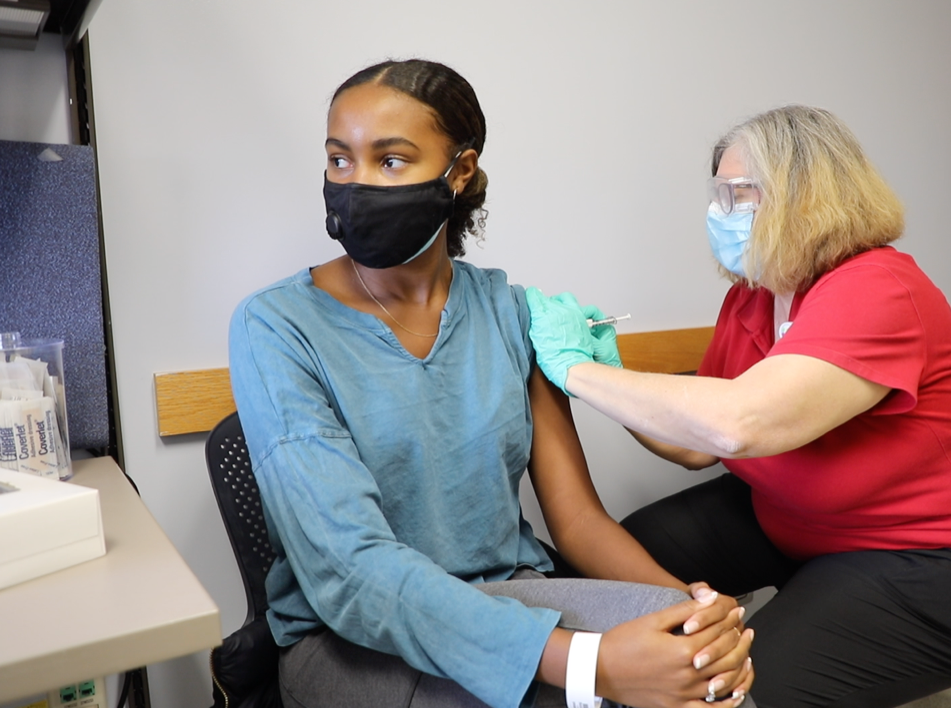
By participating, Melanie said she was helping move the vaccine forward for all kids and for women of color, but she was also helping her own school friends understand that the COVID-19 vaccine is safe.
“Most of my friends were pretty supportive. I did have a few comments … from people that didn’t trust the vaccine,” Melanie told the Cincinnati Enquirer.
In Florence, Ky., pediatrician Amanda Dropic, MD, enrolled four children in COVID vaccine trials as they opened to younger age groups, including 16-year-old Ben, 14-year-old Ty, 10-year-old Eli, and 8-year-old Lila.
“Someone has to go first,” Eli Dropic told WLWT in April 2021. “I was very nervous. I thought there are so many things that could go wrong, but there are a ton more that can go right.”
Some of the families participating in the trial found themselves targeted by anti-vaccine critics on social media. But they stood up for science. Ultimately, more than 400 children, ages 6 months to 17 years old took part.
Related Posts:
Mitigating the Mental Health Burden of COVID-19
Contributing to COVID Knowledge
One Does Not Simply…Shut Down Science
Studying the Heart in the Storm
Pandemic Prompts Dash to Build Dashboards
Exploring Intersections Between COVID-19 and Co-Morbidities
Balancing COVID Safety and Learning Needs
Anderson Center Team RACEs to Respond to COVID

Explore the full 2021 Research Annual Report
50+ Discoveries and Innovations (Enter Research Area, click on “Featured Research”)
By-the-Numbers Section Breaks Down $270M+ in Funding
Learn About Science Careers and Student Opportunities
Find Out How You Can Support Research at Cincinnati Children’s