TRAF6 Role Extends Beyond MDS to Affect Innate Immune Signaling
Research By: Daniel Starczynowski, PhD
Post Date: June 29, 2019 | Publish Date: February 2017
“Based on our paper, a number of therapeutic approaches can be tested and directed against TRAF6 and other related proteins responsible for MDS.”
—Daniel Starczynowski, PhD
A newly revealed mechanism that controls blood stem cell function may open doors to better treatments for a group of cancer-causing bone marrow disorders known as myelodysplastic syndromes (MDS).
Such a finding would be remarkable enough simply by suggesting a new way to prevent deadly cancers such as acute myeloid leukemia (AML). But the implications of this discovery appear to go beyond cancer to reveal another dimension to how cells respond to infection.
This potentially wide-scale application helps explain why the journal Nature Immunology was interested in publishing the findings from a team of scientists led by cancer biologist Daniel Starczynowski, PhD, in January 2017.
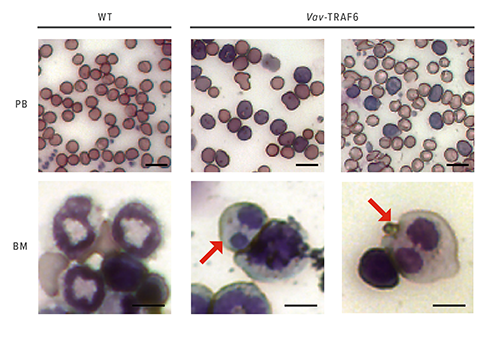
The team found that overexpression of TRAF6, a protein that normally functions as an immune sensor of pathogens, impairs blood cell formation and drives the onset of MDS.
“Based on our paper, a number of therapeutic approaches can be tested and directed against TRAF6 and other related proteins responsible for MDS,” Starczynowski says. But there’s more.
A Multifaceted Protein
Normally, in our macrophages, neutrophils and lymphocytes, toll-like receptors recognize foreign pathogens. This activates TRAF6, which in turn induces NF-kappaB and MAP kinases to mount an inflammatory response.
But TRAF6 behaves differently in MDS. In mice engineered to mimic the disease, in-depth gene expression analyses show that elevated levels of TRAF6 produce distinct changes in RNA splicing.
At one level, this finding suggests new targets for developing better treatments for MDS or AML. At another level, the paper provides new insights into how innate immune signaling pathways work in a cell.
“The implication here,” Starczynowski says, “is that not only is TRAF6 turning on certain genes, but it simultaneously communicates with the RNA splicing machinery to generate certain isoforms of genes. These isoforms may influence both the duration and the amplitude of an immune response.”
Evolving Tech
This deeper understanding how our cells fight infection has potentially broad implications for autoimmune diseases, chronic inflammatory conditions, and for cancer immunotherapy, Starczynowski says. One unusual method the researchers employed to study TRAF6 involved ubiquitin-enrichment mass spectrometry.
“The global ubiquitination screen was critical,” Starczynowski says. “When we started this project five years ago, very few people were doing these screens.”
That screening process identified 29 proteins that were ubiquitinated only in cells that overexpressed TRAF6. Of these candidates, six represented RNA-binding proteins.
Of those six proteins, one candidate stood out: hnRNPA1.
As the focus narrowed upon RNA splicing, the research team began to wonder which genes were being affected by the alternative splicing caused by TRAF6 overexpression. Several steps of analysis then revealed that hnRNPA1 was causing aberrant alternative splicing and diminished expression of the gene Arhgap1. This in turn allowed increased activity of Cdc42, a protein known to impair bone marrow function.
“About 90 percent of the time, ubiquitination is a degradation process. But in this case, it changes the function of a protein,” Starczynowski says. “That was an unexpected finding.”
A New Journey Begins
Now, Starczynowski and colleagues are working to develop small molecule compounds that can act as TRAF6 inhibitors. So far, the team has identified multiple candidate compounds and has begun pre-clinical testing. The team hopes to publish results next year.
| Original title: | Ubiquitination of hnRNPA1 by TRAF6 links chronic innate immune signaling with myelodysplasia |
| Published in: | Nature Immunology |
| Publish date: | February 2017 |
Research By