Stomach Organoid Success Advances Work to Build a Complete GI Tract on a Chip
Research By: James Wells, PhD | Kyle McCracken, MD, PhD
Post Date: June 29, 2019 | Publish Date: Jan. 12, 2017
“Now that we can grow both antral- and corpus/fundic-type human gastric mini-organs, it’s possible to study how these tissues interact physiologically, respond differently to infection and injury, and react to pharmacologic treatments.”
—James Wells, PhD
At first glance, they look like innocuous tissue samples occupying row after row of a 24-well cell culture plate. However, these blobs actually are fully functional organoids that mimic the functions of the corpus/fundus regions of the stomach—right down to producing acid and digestive enzymes.
This latest success in the fast-evolving race to grow human organ tissues in laboratory settings was impressive enough for a research team led by scientists at Cincinnati Children’s to have their study results published in the journal Nature. In fact, scientists here have made so much progress in organoid development that the medical center recently launched the Center for Stem Cell and Organoid Medicine (CuSTOM) to accelerate efforts to translate these innovations into practical technologies that may transform drug studies and clinical care for a wide range of conditions.
The new study was led by James Wells, PhD, Director of the Pluripotent Stem Cell Facility, and Kyle McCracken, MD, PhD, a former member of the Wells laboratory and now a pediatric resident at Boston Children’s Hospital. The findings come two years after the same team generated an antrum organoid that mimics the stomach’s hormone-producing region. Since 2010, researchers here also have advanced intestine and liver organoid development, with even more projects on the horizon.
“Now that we can grow both antral- and corpus/fundic-type human gastric mini-organs, it’s possible to study how these tissues interact physiologically, respond differently to infection and injury, and react to pharmacologic treatments,” Wells says.
Be it rare conditions such as Hirschsprung’s disease or common diseases like diabetes, intense demand exists for deeper understanding of GI functions and improved treatments for patients. Diseases of the stomach impact millions of people. Gastric cancer is the third-leading cause of cancer-related deaths worldwide.
If progress continues as hoped, organoid research could lead to new methods, based on fully functional human tissues instead of animal models, to test the potential toxicity of a wide range of candidates for oral medications.
At the individual patient level, custom-made organoids could help clinicians determine the most effective doses with the least risk of side effects before the patient ever receives a prescription. Eventually, lab-grown organoids may provide tissues for organ repair and transplant without the need for life-long immune suppression.
Getting there, however, will require much more investigation.
UNDERSTANDING THE BLUEPRINT
The stomach is a complex organ that requires a variety of cell types to perform its functions. So the first major challenge in developing stomach organoids was to learn much more about the molecular pathways that direct early-stage stem cells to differentiate into specific systems.
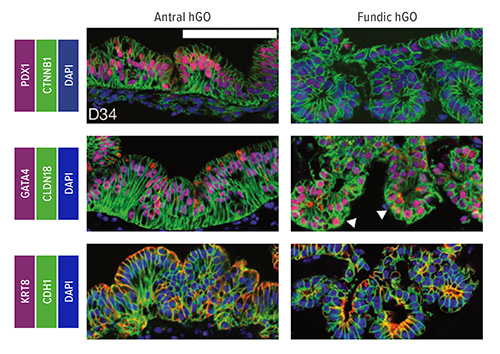
In studies involving mice, the team discovered that the WNT/b-catenin genetic pathway plays an essential role in determining whether cells become parts of the fundus or the antrum regions of the stomach. Disrupting Wnt/b-catenin signaling in mouse embryos led to conversion of fundic to antral epithelium. Meanwhile, in human pluripotent stem cells (hPSCs), b-catenin activation promoted fundic development.
Using this information, researchers manipulated the WNT/b-catenin pathway to trigger the formation of human fundus organoids from hPSCs. Study authors then further refined the process, identifying additional molecular signaling patterns that change over time to drive formation of critical cell types found in the fundus.
For example, transient inhibition of the MEK pathway at day 34 of organoid formation increased production of functional parietal cells, which secrete hydrochloric acid for digestion, the team reported. The team also observed that chief cells, which produce the digestive enzyme pepsin, mature much later in development than other cell lineages.
TRANSLATING DISCOVERY INTO APPLICATION
Overall, the organoid formation process took about six weeks. Now, researchers here are pursuing multiple plans to apply their organoid discoveries toward disease-specific research.
In one example, Wells and Yana Zavros, PhD, an associate professor at the University of Cincinnati, are exploring how fundus organoids respond to Helicobacter pylori infections. These bacteria cause chronic gastritis and stomach ulcers, and they can increase the risk of stomach cancer.
In combination with other organoids, Wells and colleagues seek to study how the body controls digestion and proper nutrient uptake, as well as a variety of medical conditions affecting the gastrointestinal tract, such as gastrointestinal motility disorders, inflammatory diseases, drug uptake studies, and harmful infections. These organoids also are conducive to studying helpful microbes (probiotics).
| Original title: | Wnt/β-catenin promotes gastric fundus specification in mice and humans |
| Published in: | Nature |
| Publish date: | Jan. 12, 2017 |
Research By







