World’s First Three-Organoid System Opens Doors for Medical Research and Diagnosis
Post Date: March 22, 2021 | Publish Date: Sept. 25, 2019

“Connecting the organs is a crucial unmet challenge to move organoid therapy into reality.”
— Takanori Takebe, MD
More than five years of painstaking research went into the project, and yet, its success came almost by accident.
Many in the field of regenerative medicine took note when a team of scientists led by first author Hiroyuki Koike, PhD, and senior author Takanori Takebe, MD, PhD, revealed in the journal Nature that they successfully produced a connected set of three organoids: the liver, pancreas and biliary ducts.
Organoids, grown from stem cells, are tiny 3D formations of human tissue that contain multiple cell types and can perform the functions of full-sized organs. Organoid experts at Cincinnati Children’s led by Jim Wells, PhD, have already grown intestines that feature nutrient-absorbing villi, stomach organoids that produce digestive acids, and others.
By themselves, human organoids already provide a sophisticated tool for research. But this advance allows scientists to study how human tissues work in concert. Eventually, organoid systems could begin reducing the need for animal-based medication studies, sharply accelerate the concept of precision medicine, and someday lead to transplantable tissues grown in labs.
“The connectivity is the most important part of this,” Takebe says. “What we have done is design a method for producing pre-organ, formation-stage tissues so that they can develop naturally. We are maximizing our capacity to make multiple organs much like our body does.”
A 5-YEAR QUEST ACHIEVES KEY GOAL
Takebe, age 32, joined Cincinnati Children’s in 2016 and holds a dual appointment at Tokyo Medical and Dental University (TMDU) in Japan. He graduated from medical school in 2011 with plans to become a liver transplant surgeon. But as he learned about the yawning gap between the supply and demand for donor organs, Takebe shifted gears to focus on organ supply.
In previous research, Takebe has demonstrated a method to produce large supplies of liver “buds,” an early-stage form of a liver organoid. He also has grown liver organoids that reflect disease states, including steatohepatitis. But Takebe says this project goes beyond his previous work.
“We noted this point in organ differentiation some time ago. But it took five years to tune up the culture system to allow this development to occur,” Takebe says.
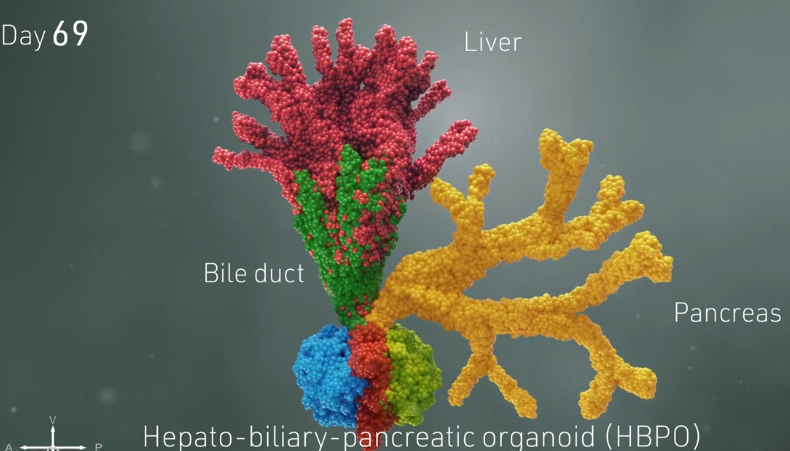
HOW THREE PROTO-ORGANS GROW IN CONCERT
The hardest parts of the process were the earliest steps.
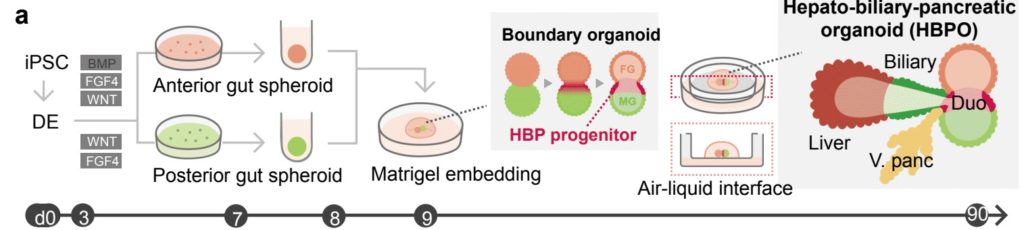
Takebe worked for many hours with colleagues at Cincinnati Children’s, including first author Hiroyuki Koike, PhD, now at Nippon Medical School in Japan, to perfect the process. They started with human skin cells, converting them back into primitive stem cells, then guiding and prodding those stem cells to form two very early-stage “spheroids” of cells loosely termed the foregut and the midgut.
These balls of cells form very early in embryonic development. In humans, they form late in the first month of gestation. In mice, they form in just 8.5 days. Over time, these spheres merge and morph into the organs that eventually become the digestive tract.
Growing these spheroids in the lab was a complex process that required using the right ingredients at the right time. Once they were mature enough—a timing step that required much work to pinpoint—then came the easier part.
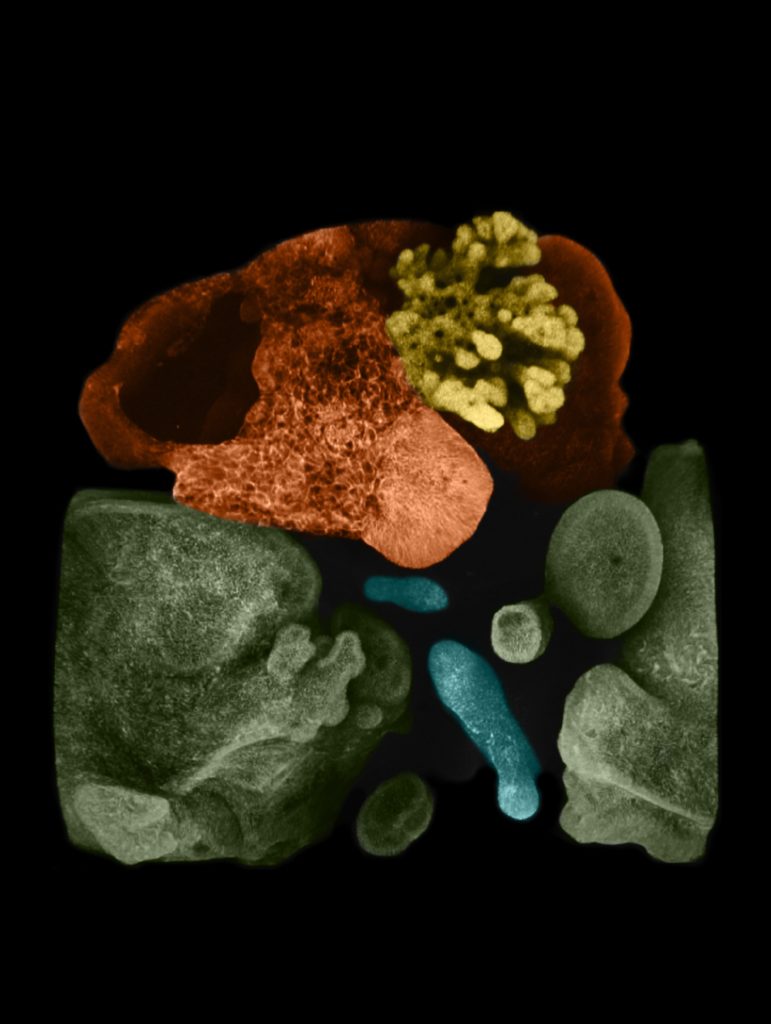
The team simply placed the spheroids next to each other in a specially arranged lab dish. The cells were suspended in a gel that’s commonly used to support organoid growth, then placed on top of a thin membrane that covered a carefully mixed batch of growth medium.
“From this point, the cells knew what to do,” Takebe says. (See video for illustration of this process.)
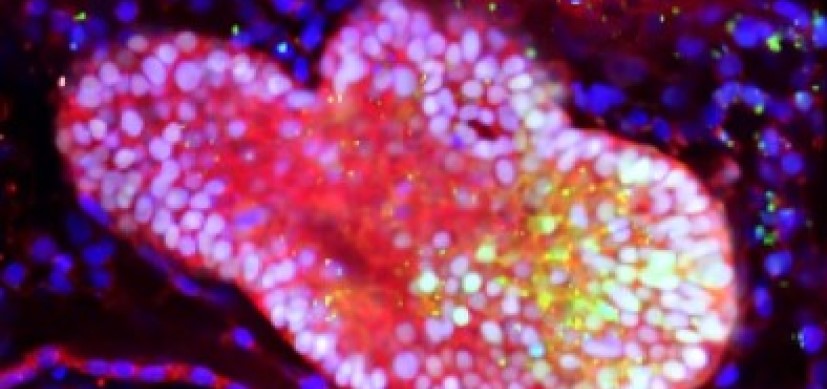
The lab team watched as cells from each spheroid began to transform upon meeting each other at the boundary between the two. They converted themselves, and each other, into more specialized cells that could be seen changing colors thanks to chemical tags the lab team had attached to the cells.
Soon, the merging, changing spheres sprouted into branches leading to new groups of cells that belonged to specific organs. Over 70 days, these cells multiplied into more refined and distinct cell types. Ultimately, the organoids began processing bile acids as if they were digesting and filtering food.
“This was completely unexpected. We thought we would need to add ingredients or other factors to push this process,” Koike says. “Not trying to control this biological process led us to this success.”
WHAT DOES THIS ADVANCE MEAN?
Aaron Zorn, PhD, Director of the Center for Stem Cell and Organoid Medicine (CuSTOM) at Cincinnati Children’s, says this advance will be useful in multiple ways.
“The real breakthrough here was to be able to make an integrated organ system,” Zorn says. “From a research perspective this is an unprecedented opportunity to study normal human development.”
However, Takebe and colleagues were able to grow these organoids only so far. More work is
needed to grow tissues large enough to be useful in human transplantation. The organoids also need to be integrated with other cell lines that form blood vessels, connective tissues, and so on.
“Current liver regenerative medicine approaches suffer from the absence of bile duct connectivity,” Takebe says. “While much work remains before we can begin human clinical trials, our multi-organoid transplant system is poised to solve this issue.”

More Top 5 Achievements from the FY20 Research Annual Report:
-
Dose Escalation Sharply Improves Hydroxyurea Benefit for Children with Sickle Cell Anemia
-
Cardiac Stem Cell Therapy Improves Scar Formation Rather Than Prompting Cardiomyocyte Regeneration
-
Clinical Trial Success Caps Long Journey for HLH Treatment
-
Single Cell Approach Reveals Impact of Disease-Causing Gene Mutations
Read the full report
Request a PDF
| Original title: | Modelling human hepato-biliary-pancreatic organogenesis from the foregut–midgut boundary |
| Published in: | Nature |
| Publish date: | Sept. 25, 2019 |