Clinical Trial Success Caps Long Journey for HLH Treatment
Post Date: March 22, 2021 | Publish Date: May 2020

“For the first time we have a truly targeted way to treat HLH and a drug with very low toxicity.”
—Michael Jordan, MD
The disease hemophagocytic lymphohistiocytosis (HLH) still has no cure. But now it has an approved treatment that appears to help most patients live long enough and well enough to receive crucial stem cell transplants.
The efficacy of the monoclonal antibody emapalumab (Gamifant) was detailed in clinical trial results published in The New England Journal of Medicine, The multi-center study was co-authored by Michael Jordan, MD, a member of the Divisions of Immunobiology and Bone Marrow Transplantation and Immune Deficiency, and Alexei Grom, MD, Research Director, Division of Rheumatology.
The biologic, when administered with dexamethasone, achieved its goal of tamping down runaway inflammatory activity while demonstrating less toxicity and fewer side effects than other treatments, such as steroids and chemotherapy.
The NEJM paper delved into data from a clinical trial that began in 2013 and officially ended in 2017, although some patients were treated with the drug after the cutoff date. Based on the initial data from the trial, the U.S. Food and Drug Administration approved emapalumab in 2018—the first-ever drug approved specifically for HLH.
The initial trial results suggested that clinicians should consider the drug as the standard of care for “second line” treatment of HLH. But the extended findings also suggest a potential benefit for the drug as a first-line therapy, Jordan says.
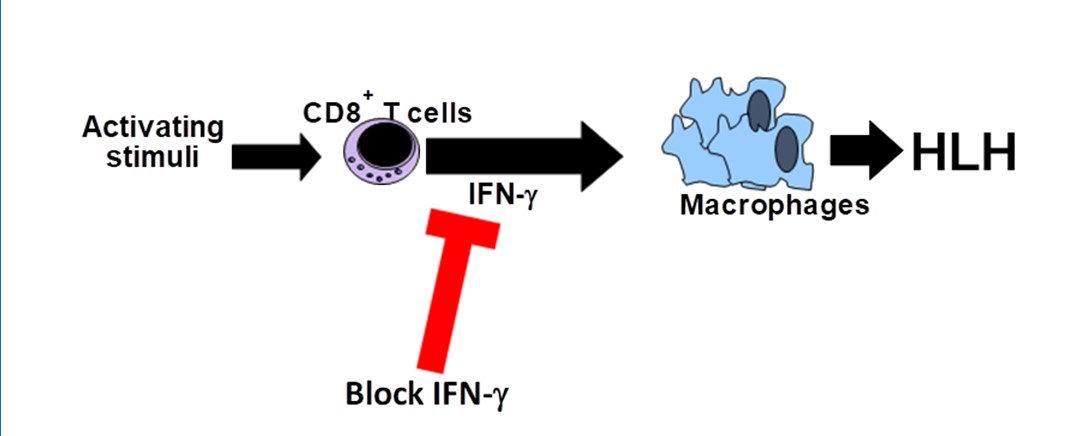
STOPPING AN INFLAMMATION TRAIN
HLH is a rare but deadly childhood disease that overstimulates the immune system and causes hyperinflammation, which leads to widespread organ and tissue damage. Clinicians find about 1.2 cases per 1 million individuals per year. For many years, the mortality rate has been about 40%.
Jordan, has been working for years to find a treatment specific to HLH that can block the runaway inflammation. A key step in that effort occurred in 2004, when Jordan’s mouse study, published in the journal Blood, revealed that the HLH disease process relies on high levels of the protein interferon gamma (IFNg).
Now, emapalumab has shown its ability to block IFNg, which in turn offers improved odds for more children to live with HLH as more of a chronic disease instead of an early cause of death.
ENCOURAGING RESULTS
The phase 2–3 clinical trial was performed at 14 sites in Germany, Italy, Spain, the United Kingdom, and the United States. Jordan led the project in conjunction with Franco Locatelli, MD, at the University of Rome.
Overall, 34 patients received emapalumab, including 27 who had received other unsuccessful treatments and seven patients who had not been treated in any other way.
The eight-week treatment began with an emapalumab dose of 1 mg per kilogram of body weight every three days, which was increased over time up to as high as 10 mg per kilogram. Twenty-six patients completed the study.
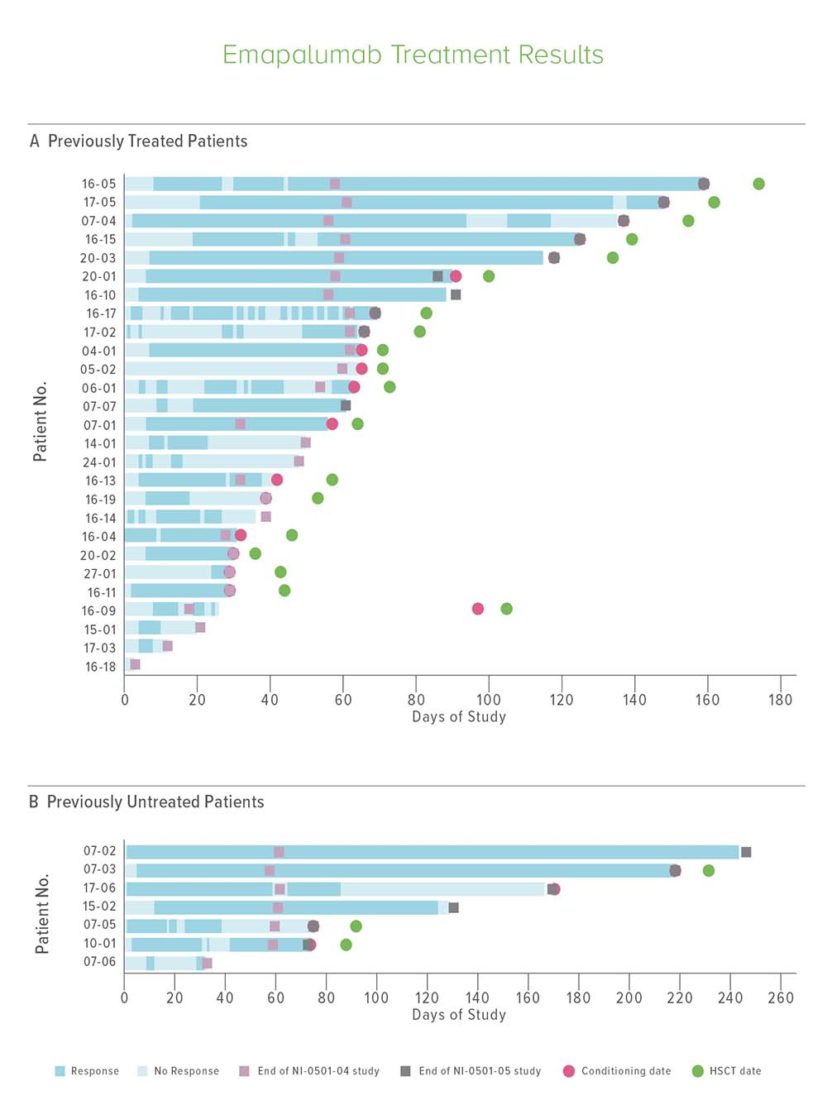
Investigators found that 63% of the previously treated group and 65% of previously untreated patients responded to emapalumab. This included seven patients who experienced “complete responses,” defined as normal spleen and no fever, cytopenia, hyperferritinemia or other abnormalities attributable to hemophagocytic lymphohistiocytosis.
Of the initial 34 patients, 22 ultimately received hematopoetic stem cell transplant with one-year survival rates exceeding 70%.
“This is a very important advance. For the first time we have a truly targeted way to treat HLH and a drug with very low toxicity,” Jordan says.
The clinical trial was funded in large part by the drug maker. However, the research that led up to the trial was funded by a mix of government grants and philanthropy.
In fact, participants from several years of 700-mile bike rides from Mississippi to Cincinnati raised more than $1 million to support the work.

HUNT UNDERWAY FOR GENE THERAPY
At the HLH Center of Excellence, Cincinnati Children’s has assembled a team of specialists and scientists who continue to work to improve HLH outcomes.
In addition to the NEJM study results, the team has developed an HLH gene chip for rapid, precise diagnosis and has begun preclinical studies of a potential gene therapy for PRF1 deficiency.
For emapalumab, more study is needed to track longer-term effectiveness, including to determine whether the drug can reduce or delay the need for stem cell transplants.
“My goal for our work over all these years has been to use scientific exploration and practical medical approaches to make a difference in the lives of these children,” Jordan says. “It’s been a privilege to help develop this idea from an unexpected laboratory discovery to an approved medicine.”
LEARN MORE ABOUT HLH

More Top 5 Achievements from the FY20 Research Annual Report:
-
Dose Escalation Sharply Improves Hydroxyurea Benefit for Children with Sickle Cell Anemia
-
World’s First Three-Organoid System Opens Doors for Medical Research and Diagnosis
-
Cardiac Stem Cell Therapy Improves Scar Formation Rather Than Prompting Cardiomyocyte Regeneration
-
Single Cell Approach Reveals Impact of Disease-Causing Gene Mutations
Read the full report
Request a PDF
| Original title: | Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis |
| Published in: | NEJM |
| Publish date: | May 2020 |






