Clinical Science: The Art of Moving Discovery from Bench to Bedside
Post Date: January 24, 2024 | Publish Date:

Clinical research often can be a world of its own, dominated by the involvement of pharmaceutical companies, institutional review boards, federal regulations, and the challenges of overcoming ongoing distrust of the medical system to recruit study participants across the full range of human demographic variation. Moving from lab dishes and animal models to humans in need takes everything about science to a higher level. These stories are just some of the many difference-making examples of clinical research led by faculty here.
Be it carrying out classic three-phase clinical drug trials or following other closely regulated avenues of human subjects research, investigators at Cincinnati Children’s are leaders in translating lab breakthroughs into real treatments that improve outcomes.
World’s 1st FLASH Proton Therapy Trial Shows Promise, Earns Accolades
“The unique FLASH research at our proton therapy center in Cincinnati has far-reaching implications for how we treat cancer in kids and adults,” says John Perentesis, MD, research director for the Proton Therapy Center, director of the Division of Oncology, and co-director of the Cancer and Blood Diseases Institute at Cincinnati Children’s.
“It’s important because radiation is one of the most effective tools for treating cancer and is used for nearly half the cancer patients in the U.S.,” Perentesis says. “FLASH holds promise to become a paradigm-shifting technology by potentially providing more effective cancer treatment with fewer side effects. It is the top priority area for new therapy development at our proton therapy center–with research underway in brain tumors and DIPG, sarcomas, neuroblastoma, and lymphomas.”

The impressive initial results of this advanced form of radiotherapy were presented Oct. 23, 2022, during the annual meeting of the American Society for Radiation Oncology (ASTRO), and published Oct. 24, 2022, in JAMA Oncology.
The FAST-01 study was led by John Breneman, MD, medical director of the Proton Therapy Center. Emily Daugherty, MD, a radiation oncologist at the UC College of Medicine and Cincinnati Children’s shared the results at ASTRO. Anthony Mascia, PhD, chief physicist at the Proton Therapy Center, was the principal author of the landmark JAMA Oncology paper.
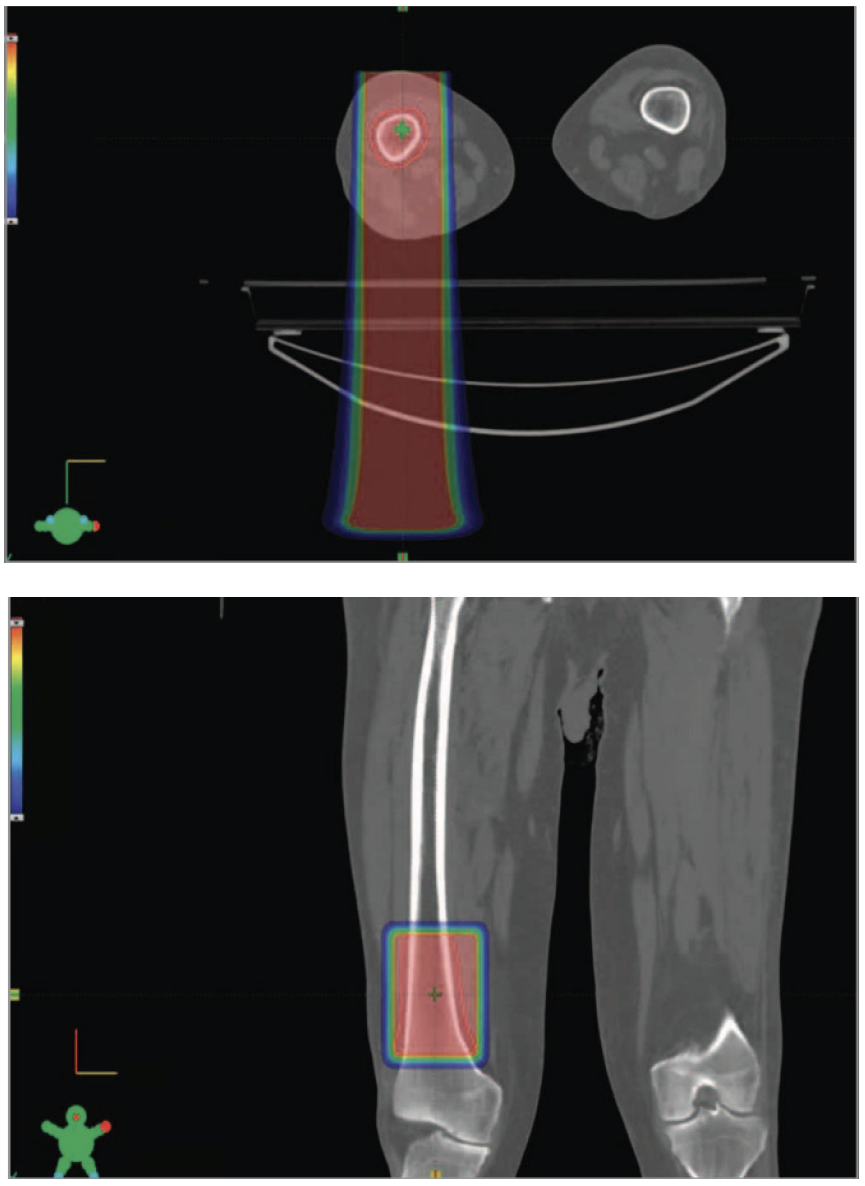
The study evaluated 10 adults (ages 27-81) who underwent palliative FLASH therapy to bone metastases in their extremities. Participants reported pain relief in eight of 12 treated sites treated and reported feeling no pain at all in six of 12 sites. Now, the race is on to evaluate this technology for treating more types of cancer across many more age groups.
While a version of proton therapy that requires numerous return visits for repeated dosings has been used beneficially for several years, FLASH is new. This novel approach delivers a high dose of protons in less than one second.
Read more about the FLASH study
Massive upfront investment paying off
The development of FLASH technology happened in our region because of a forward-thinking collaboration between Cincinnati Children’s, the University of Cincinnati, UC Health, and Varian, the company that makes proton therapy equipment.
Not only did Cincinnati Children’s invest $120 million in 2016 to build the Proton Therapy Center along I-75 in Liberty Township north of Cincinnati, the project included $24 million for a one-of-a-kind research facility. That facility includes a fully operational proton treatment room dedicated exclusively for research along with integrated laboratories.
When the facility opened, Perentesis predicted: “This center will become a national anchor for particle-based cancer research.”
A second FLASH clinical trial was launched in March 2023. And in December 2022, the editors of Physics World ranked FLASH proton therapy among the magazine’s “Top 10 Breakthroughs of the Year for 2022” – a list that also celebrates stunning images from the James Webb Space Telescope and the potentially planet-protecting success of NASA’s Double Asteroid Redirection Test (DART) mission.
Two-Decade Journey Leads to 1st FDA-Approved Drug for EoE
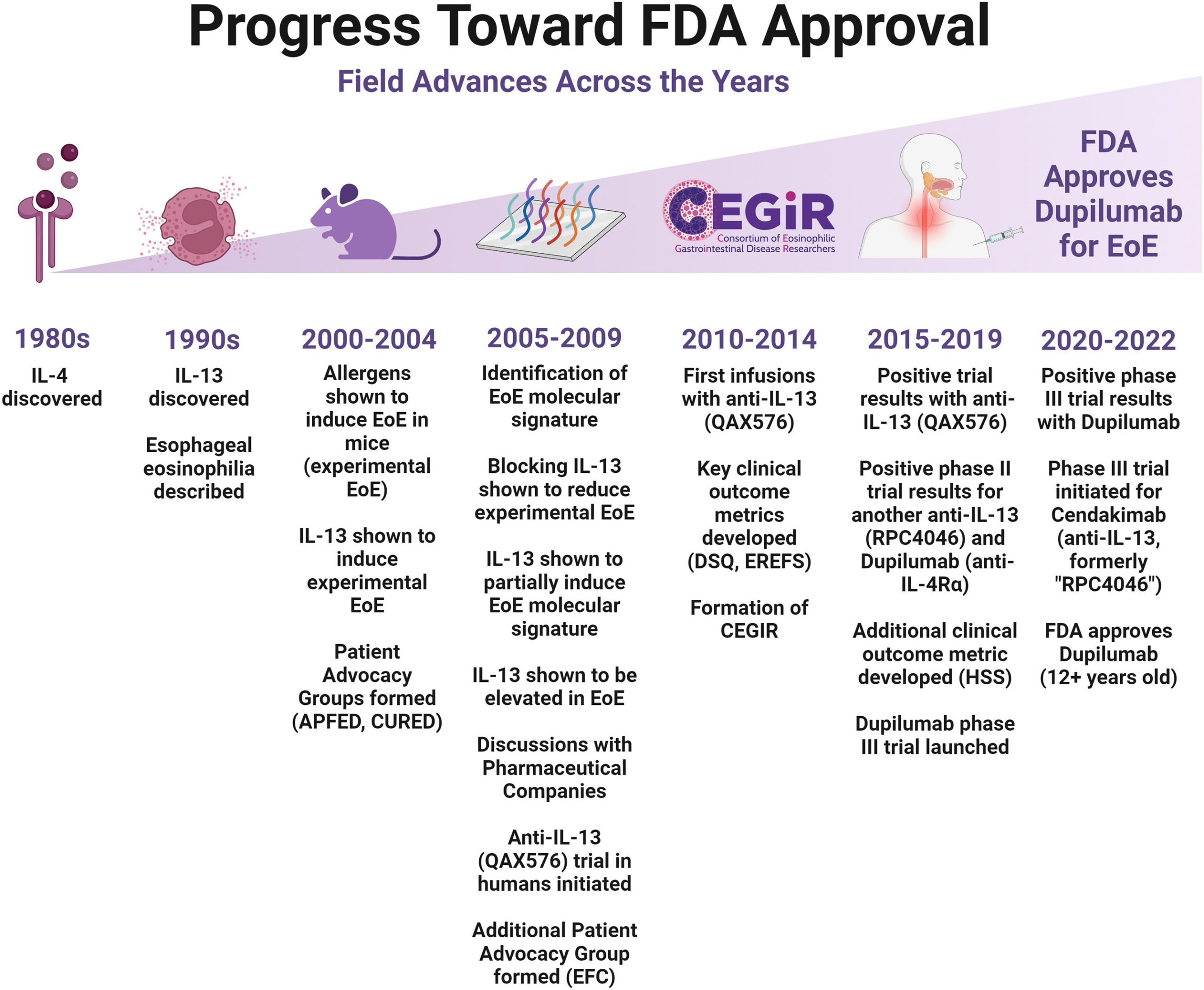
Few other people in modern medicine can be as closely tied from the discovery of a significant childhood disease through to the creation of an important treatment as Marc Rothenberg, MD, PhD, has been linked to the study of eosinophilic disorders.
In an era of collaborative team research, Rothenberg certainly has not been the sole innovator in the quest to define and understand eosinophilic esophagitis. But since EoE emerged in the 1990s as the first of a family of related conditions, no single investigator has played a bigger leading role.

Rothenberg’s team has produced more than 500 peer-reviewed articles about eosinophilic disorders in publications including The New England Journal of Medicine, Nature Genetics, Gastroenterology, The Journal of Allergy and Clinical Immunology, and more. He is principal investigator for the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) a national network funded by the Rare Diseases Clinical Research Network (RDCRN). He has co-established fundraising organizations to support discovery and care. He also has launched multiple social media channels where he speaks directly with families coping with these conditions and with a network of scientists in the field.
All of that work reached a pinnacle in 2022, when the US Food and Drug Administration approved the monoclonal antibody dupilumab as the first treatment specifically for managing EoE.
Rothenberg was co-first author of the study in The New England Journal of Medicine that laid out the Phase III clinical trial findings from evaluating the impact of dupilumab in adolescents and adults with EoE. He also played a central role in a 1-year follow-up study published in The Lancet Gastroenterology & Hepatology.
Margaret Collins, MD, Division of Pathology, was a co-author and significant contributor to both of these dupilumab studies.
Now, dupilumab is a fundamental part of treatment plans for EoE in medical centers worldwide. And Rothenberg’s leadership has been widely recognized. He was inducted to the National Academy of Medicine in October 2022. In 2023, he also received the Distinguished Scientist Award from the American Academy of Allergy, Asthma & Immunology and a Daniel Drake Medal, the highest honor awarded by the University of Cincinnati College of Medicine.
Driving Safer Outcomes for Teens with ADHD
Great medicine isn’t always about medications. An innovative clinical study led by Jeffery Epstein, PhD, and colleagues in the Division of Behavioral Medicine & Clinical Psychology revealed that better drivers’ training for teens with attention-deficit/hyperactivity disorder (ADHD) can save lives.

The study, published Dec. 1, 2022, in The New England Journal of Medicine, details outcomes of the FOCAL+ training program, which used a mix of simulation training and real-world experiences to help teens with concentration challenges concentrate on the road.
After participants completed the one-month training program, the researchers outfitted the teens’ vehicles with eye-monitoring cameras and tracked their driving for a year. The teens who were warned during their training about their overly-long side glances had 40% fewer crashes and near-misses compared to a control group of teens who did not receive the warnings.
Now, Cincinnati Children’s has made the training program available to regional families outside the clinical study. The program leaders also are working on plans to expand the program beyond the Cincinnati region.

SPHERE Trial Validates a Low-Cost Sickle Cell Treatment Halfway Around the Globe
Stroke risk is one of the most severe potential outcomes of undertreated sickle cell disease. In the United States and other wealthy nations, a variety of treatments from frequent blood transfusions to emerging gene therapies are available to help limit this risk.

But in Africa, where sickle cell disease is far more common, many of those care approaches are too expensive for families and often beyond the capacity of local hospitals in low-resource nations to provide even when families can pay.
In March 2023, researchers from Cincinnati Children’s teamed up with doctors in Tanzania to demonstrate a way to overcome such barriers. In a paper published in The Lancet Haematology the investigators show that treating children with low-cost hydroxyurea while monitoring blood flow velocity using relatively simple ultrasound screening can sharply reduce stroke risk.
Cincinnati Children’s co-authors included corresponding author Luke Smart, MD, and collaborators Russell Ware, MD, PhD, Adam Lane, PhD, Susan Stuber, MS, and Teresa Latham, MA, PCC-S.
Applying Green Eyeshades to Rare Disease Studies
Limited patient populations can make clinical trials for rare disease treatments slow and costly. However, applying advanced statistical methods to historical data can speed the process up, according to an impressive study published by experts in the Division of Biostatistics and Epidemiology at Cincinnati Children’s.


Investigators re-analyzed data from the MILES trial led by Francis McCormack, MD, at the University of Cincinnati—a highly successful study that resulted in the FDA’s approval of sirolimus for treating lymphangioleiomyomatosis (LAM).
By using select “historical” data instead of hunting for concurrent random controls, the study could have obtained the same results in five months less time, according to research led by first author Nusrat Harun, PhD, and senior author Maurizio Macaluso, MD, DrPH.
This approach could be used in many more studies, Macaluso says. And when children are battling conditions that have few or no effective treatments, every day shaved off the study process could be vital.







