World’s 1st FLASH Proton Therapy Clinical Trial Shows Promise
Post Date: October 24, 2022 | Publish Date: Oct. 24, 2022
Cancer & Blood Diseases Institute
Oncology | Top Scientific Achievement

Scientists from Cincinnati Children’s/UC Medical Center Proton Therapy Center Led Study Demonstrating Feasibility, Safety of Ultra-Fast Cancer Treatment
Research findings from the world’s first clinical trial of proton FLASH therapy in humans, which was conducted at the Cincinnati Children’s/University of Cincinnati Medical Center Proton Therapy Center, indicate the clinical feasibility and preliminary efficacy and safety of an experimental treatment for cancer patents that delivers radiation therapy at ultra-high dose rates in less than 1 second.
Results of the FAST-01 trial (FeAsibility Study of FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases) were presented Oct. 23, 2022, during the annual meeting of the American Society for Radiation Oncology (ASTRO).
How the clinical trial got started

The research findings were published Oct. 24, 2022, in JAMA Oncology.
“The unique FLASH research at our proton therapy center in Cincinnati has far-reaching implications for how we treat cancer in kids and adults,” says John Perentesis, MD, research director for the Proton Therapy Center, director of the Division of Oncology, and co-director of the Cancer and Blood Diseases Institute at Cincinnati Children’s. “It’s important because radiation is one of the most effective tools for treating cancer and is used for nearly half the cancer patients in the U.S. FLASH holds promise to become a paradigm-shifting technology by potentially providing more effective cancer treatment with fewer side effects. It is the top priority area for new therapy development at our proton therapy center–with research underway in brain tumors and DIPG, sarcomas, neuroblastoma, and lymphomas.”
The Proton Therapy Center, which opened in 2016 on the Liberty Campus of Cincinnati Children’s, incorporates a $24 million, one-of-a-kind research facility. It includes a fully operational proton treatment room dedicated exclusively for research along with integrated laboratories.
The unique capabilities of the research center were instrumental in Cincinnati developing the world’s first clinical trial of FLASH proton therapy. In addition, children and adults from around the world seek treatment for more than 30 types of cancer in two clinical therapy rooms at the center.
The FAST-01 study was led by John Breneman, MD, medical director of the Proton Therapy Center, and Emily Daugherty, MD, a radiation oncologist at the UC College of Medicine and Cincinnati Children’s. Daugherty shared the results at the ASTRO meeting in San Antonio.
Anthony Mascia, PhD, chief physicist at the Proton Therapy Center, is the principal author of the landmark JAMA Oncology paper.
The clinical trial was designed by experts at Cincinnati Children’s, UC and Varian, the Siemens Healthineers company that built the device.
“Based on clinical workflow metrics, treatment efficacy, and safety data, researchers concluded that ultra-high dose rate proton FLASH therapy is feasible in a clinical setting,” Varian reported in an Oct. 24 news release. “Data surrounding the first experience in humans showed the desired therapeutic benefit for most trial participants and no significant toxicity.”
The clinical trial involved the investigational use of Varian’s ProBeam particle accelerator modified to enable and control the delivery of radiation therapy at ultra-high dose rates, allowing the entire dose to be delivered in less than 1 second.
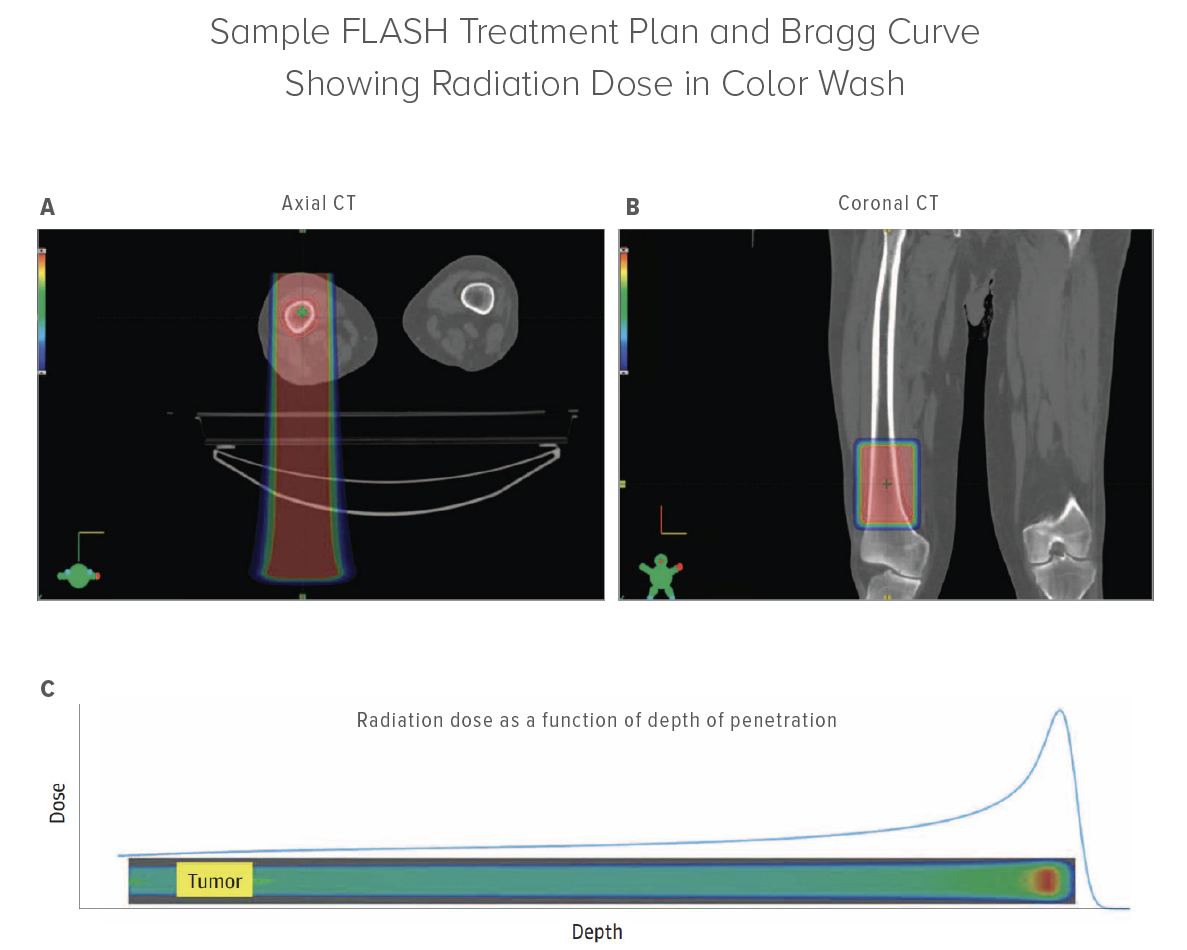
FAST-01 enrolled 10 participants between the ages of 27 and 81. They underwent palliative FLASH therapy to bone metastases in the extremities. Endpoints were evaluated by clinical workflow feasibility, treatment-related side effects, and efficacy of treatment as assessed by measuring pain relief of trial participants. Eight of 12 sites (67%) treated had pain relief, and six of 12 sites had a complete response (no pain). All adverse events were mild and consistent with those expected from conventional radiotherapy.
A second human trial of FLASH therapy is underway at the Proton Therapy Center.
Learn more about the new trial
View more discoveries from 50 research divisions and areas
Return to the 2023 Research Annual Report main features
| Original title: | Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases The FAST-01 Nonrandomized Trial |
| Published in: | JAMA Oncology |
| Publish date: | Oct. 24, 2022 |