Characterizing Eosinophilic Duodentitis
Research By: Tetsuo Shoda, MD, PhD | Marc Rothenberg, MD, PhD
Post Date: January 20, 2023 | Publish Date: Dec. 30, 2022
Eosinophils live in the segments of the gastrointestinal tract below the esophagus, including the stomach and small and large intestines, helping us monitor and combat any outside threats that enter our bodies through our mouths. However, high levels of eosinophils, called eosinophilia, occur in diseases such as in eosinophilic gastrointestinal diseases (EGID).
But how many eosinophils are too many? What number of eosinophils separates health from disease? How does a high level of eosinophils in the top part of the small intestine (duodenum), called eosinophilic duodenitis (EoD), relate to other gastrointestinal conditions, such as celiac disease, inflammatory bowel disease and EGID?
These are questions that physicians and researchers at Cincinnati Children’s explored for the enigmatic condition of EoD. The findings, published online Dec. 30, 2022, in the Journal of Allergy and Clinical Immunology, are the first to molecularly analyze EoD.
“EoD is characterized by non-specific gastrointestinal symptoms and increased duodenal eosinophils. Clinically, we believed that it may be in the EGID spectrum,” says Tetsuo Shoda, MD, PhD, Division of Allergy and Immunology at Cincinnati Children’s. “We sought to define the genes and pathways involved in the pathology of EoD in order to better understand the disease and how we might treat it and to establish thresholds of eosinophil levels to improve diagnosis.”
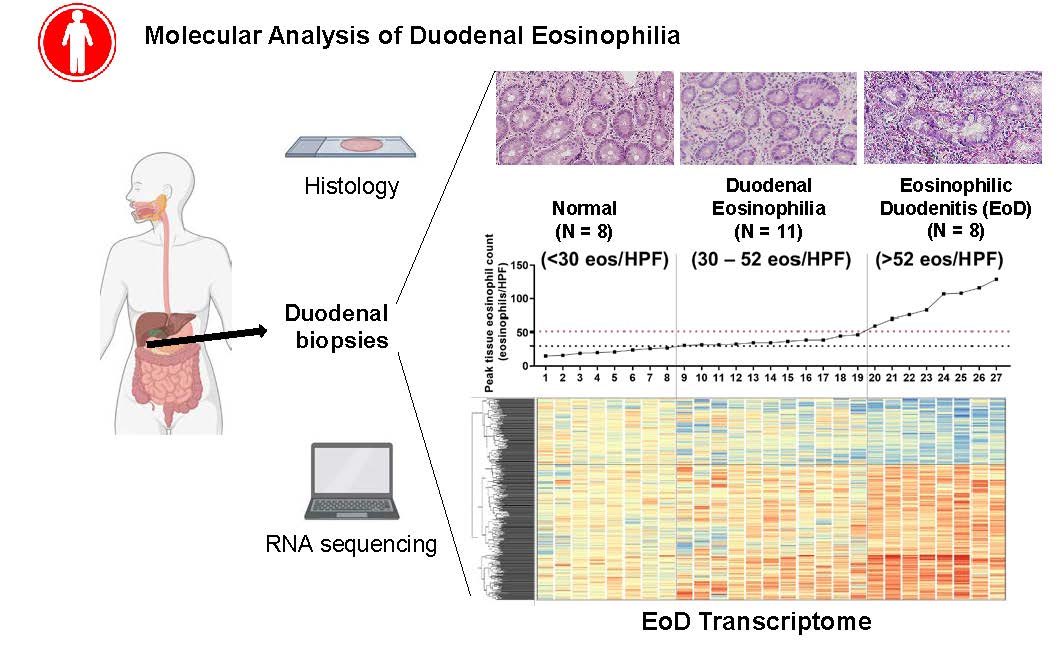
The team identified the gene expression signature of EoD, called the EoD transcriptome, that emerges at eosinophil levels of 50-60 peak eosinophils per high-power microscopic field of duodenal tissue biopsies. Notably, the EoD transcriptome signature established EoD as part of a spectrum of upper EGID associated with type 2 immunity, like the EGIDs eosinophilic esophagitis (EoE) and eosinophilic gastritis (EoG), and distinct from the EGID eosinophilic colitis (EoC) and from celiac disease.
“This key first characterization of EoD provides a basis for improving diagnosis and treatment,” says senior author Marc Rothenberg, MD, PhD, director, Division of Allergy and Immunology and the Center for Eosinophilic Diseases at Cincinnati Children’s and principal investigator of the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). “I look forward to applying these findings to clinical practice and to delving deeper into understanding this understudied condition and related EGID.”
ABOUT THE STUDY
In addition to Shoda and Rothenberg, Cincinnati Children’s co-authors included Margaret Collins, MD, Vincent Mukkada, MD, and Philip Putnam, MD.
This work was supported by NIH grant K99/R00 AI158660 (to TS) and the Digestive Health Center (P30 DK078392 [Gene and Protein Expression Core]) and by NIH grants R01 AI045898, R01 AI124355, and U19 AI070235; the Campaign Urging Research for Eosinophilic Disease (CURED); and the Sunshine Charitable Foundation and its supporters, Denise A. Bunning and David G. Bunning] (to MER).
Read more about research of the Shoda Lab and Rothenberg CURED Lab
| Original title: | Molecular Analysis of Duodenal Eosinophilia |
| Published in: | The Journal of Allergy and Clinical Immunology |
| Publish date: | Dec. 30, 2022 |
Research By

The Shoda Lab at Cincinnati Children’s is working to identify the key genes and genetic regulatory mechanisms that predispose patients to eosinophilic conditions, especially eosinophilic gastrointestinal diseases (EGID).

The Rothenberg CURED Research Laboratory, supported by the Campaign Urging Research for Eosinophilic Diseases (CURED), is focused on elucidating the mechanisms of allergic responses, especially in mucosal tissues such as the gastrointestinal tract and lung.