Basic Science: Chasing the Undiscovered
Post Date: January 24, 2024 | Publish Date:

There’s nothing “basic” about basic research. Many of the scientists funded through our Research Foundation are seeking answers to the most elusive and fundamental questions of biology. Their work seeks to define how human development occurs in hopes of pinpointing where the complex mix of genetic programming, molecular signaling, and a lifetime of interactions with the world around us can go wrong, giving rise to disease and dysfunction. We don’t know what we don’t know—until we ask the right questions.
From secrets of brain development to mysteries of somite segmentation, scientists at Cincinnati Children’s pursue deeper understanding of the human condition

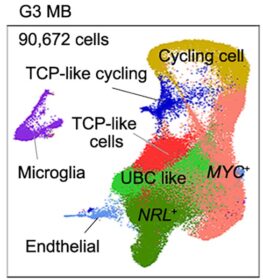
Single-Cell ‘Atlas’ Provides Roadmap for Battling Brain Tumors
Overall, the five-year survival rate for people with medulloblastoma is about 72%. However, about 27% of people with this cancer (most commonly children ages 4 to 16) develop “Group 3” aggressive medulloblastomas, which have five-year survival rates of only 20 to 30%.
Now a basic science breakthrough that produced a new atlas of human fetal brain development has opened new doors to better treatments for this deadly form of cancer. And that might be just the beginning of the potential benefits.
Detailed findings were published in December 2022 in Nature.
“While this study focuses on medulloblastoma, the new atlas will help accelerate understanding of other conditions that result from disruptions in healthy early brain development, such as autism, attention deficit-hyperactivity disorder (ADHD), developmental dyslexia, and pediatric cerebellar damage,” says senior author Qing Richard Lu, PhD, scientific director of the Brain Tumor Center in our Division of Experimental Hematology and Cancer Biology.
The Power of Collaboration
Producing the atlas required a team of nearly 40 scientists working across continents for more than three years. Collaborators included several members of our Cancer and Blood Diseases Institute at Cincinnati Children’s, plus colleagues at Northwestern University in Illinois, The Hospital for Sick Children in Toronto, the Cancer Research UK Cambridge Centre, and Fudan University, Shanghai Jiao Tong University, and Westlake University in China.
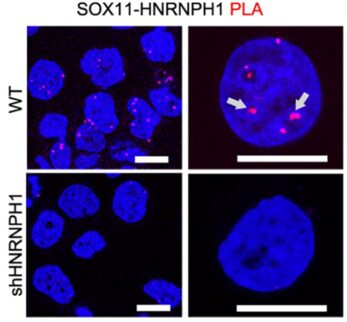
The atlas helped researchers identify a key collection of progenitor cells linked to aggressive medulloblastomas. These cells are rarely found in mice, and only temporarily present in the human brain during fetal development. However, deviations in healthy formation of these cells appear to lead to excessive MYC gene activation, driving tumor formation.
“Higher proportions of the cerebellar progenitor cells in Group 3 medulloblastomas might contribute to the difference in survival outcomes,” Lu says.
Setting a New Course for Future Studies
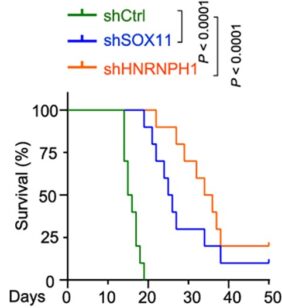
The differences the team found between mouse and human cells help explain why previous mouse model systems have not sufficiently reflected the human experience with human medulloblastoma. However, when using a new mouse model, the team found that eliminating or sharply reducing the activity of either of two abundant genes in the progenitor cells–SOX11 or HNRNPH1–reversed tumor formation triggered by hyperactivity of the cancer-driving MYC gene.
More research is needed to translate these findings into potential human therapy, but this basic research to detail how the brain develops may have revealed a “targetable vulnerability” of aggressive medulloblastoma tumors.
(Click arrows to view more slides)
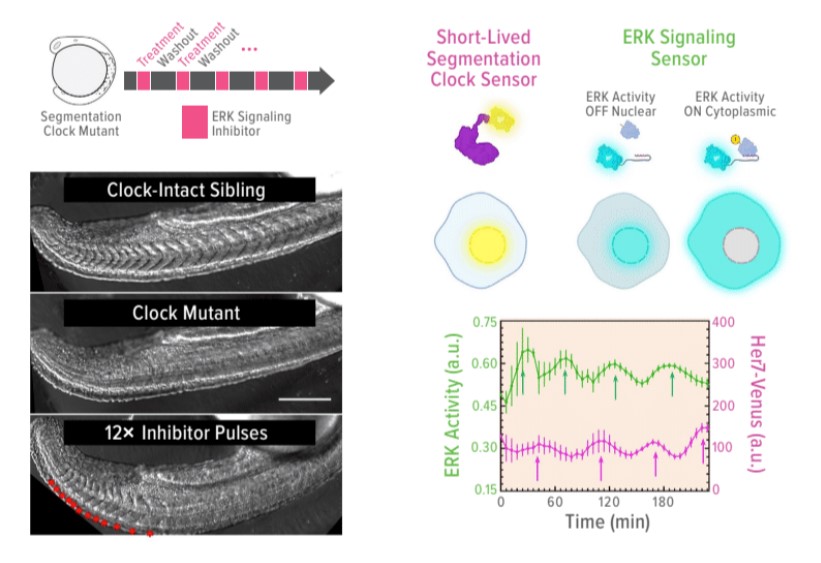
Can Off-Beat Clocks of Early Development be Reset?
Developmental biologists have devoted years to documenting the exquisitely complex dance of stem cells and progenitor cells as they seek their mature fates and assume their specific roles as bones, tissue, nerves and so on. Of special interest to many scientists has been understanding how organisms manage to make matching, repeating parts grow on opposite sides of the body.
Much about the process of somite segmentation remains unknown.


But after conducting an extensive series of experiments involving zebrafish, a research team led by M. Fethullah Simsek and Ertuğrul Özbudak, PhD, in our Division of Developmental Biology, shed new light on the clockwork mechanisms driving the formation of repetitive structures. Their eye-opening findings were published in December 2022 in Nature.
So how do specific cells at specific locations “know” it’s time to form a new segment?
Zebrafish, which offer clear embryos for easier examination and can be produced quickly in large numbers, make an ideal model for exploring the genetic combinations and signaling mechanisms involved in segmentation. In this study, the researchers pinpointed two key elements of the process: oscillating clock genes Her1 and Her7, which change the levels of double-phosphorylated Erk (ppErk) in the developing zebrafish. The periodic lowering of ppERK levels prompts new segments to form.
But what happens when the clock genes malfunction? This can lead to severe skeletal malformations, as shown in the study. However, the body has its backup systems. When the primary clock genes fail, other clock genes can kick in to provide the pulsing signals needed to establish somite boundaries. In fact, the need for specific clock genes may not be required at all.
“With further experimentation, we found that artificially triggering pulsatile inhibition of the ppErk gradient can fully substitute for the role of the clock in zebrafish development,” Özbudak says. “This finding could have a variety of applications, including new methods to correct dysfunctional developmental and inducing balanced formation of organoid tissues that involve segmentation.”
Protecting Damaged Livers from Immune System Attack
Children born with rare liver diseases like biliary atresia ultimately need an organ transplant to survive because the main duct that carries bile from the liver to the intestine fails to function. But what if there was a way to protect bile ducts from the inflammatory injury associated with these diseases?



A study led by first authors Tiffany Shi, an MD/PhD student and Astha Malik, PhD, along with corresponding author Alexander Miethke, MD, suggests that such protection may be possible. Their findings were published in December 2022 in Science Translational Medicine.
While damaged bile ducts can cause toxic bile acid build-up in the liver, this research team discovered that the immune response to damaged tissues also significantly contributes to organ damage. They went on to demonstrate in mouse models of sclerosing cholangitis that this response can be controlled by activating the farnesoid X receptor gene (FXR) within macrophage cells. FXR activation reduced production of inflammatory cytokines and constrained unwanted T cell multiplication. This protective effect was further confirmed in studies of immune cells from patients with liver diseases.
Sometimes, Treatment Can Be Sex-Dependent
Status epilepticus affects about 20 per 100,000 children per year, with mortality rates reaching up to 30%. The condition is associated with a striking increase in circulating corticosteroids. However, whether this increase is pathological, unimportant, or even beneficial, is not clear.


Now, in a study in mice, experts at Cincinnati Children’s report that the severity of status epilepticus can be controlled by reducing the number of glucocorticoid receptors (GRs) in certain brain cells. By directly knocking out these receptors in brain regions associated with seizures, the investigators isolated brain-specific effects of corticosteroids. They also found that the roles GRs play during status epilepticus change as time passes and may depend upon the sex of the patient.
The study was led by first author Kimberly Kraus, PhD, and corresponding author Steve Danzer, PhD. Six other colleagues from Cincinnati Children’s and the University of Cincinnati were co-authors.