Systemic FXR Agonists Emerge as Potential Treatment for Cholestatic Liver Diseases
Research By: Tiffany Shi | Astha Malik, PhD | Alexander Miethke, MD
Post Date: December 14, 2022 | Publish Date: Dec. 14, 2022
Gastroenterology, Hepatology and Nutrition | Top Scientific Achievement

Experts at Cincinnati Children’s find early promising signs from a new approach that targets how macrophages respond to biliary atresia and primary sclerosing cholangitis
When children are born with rare liver diseases like biliary atresia, they ultimately need an organ transplant to survive because the main duct that carries bile from the liver to the intestine fails to function. But what if there was a way to protect bile ducts from the inflammatory injury associated with these diseases?
Now a study led by liver disease experts at Cincinnati Children’s suggests that liver function can be preserved–in mice–by better controlling how the immune system’s macrophages respond to diseased tissue. Their findings, led by first authors Tiffany Shi, an MD/PhD student at the University of Cincinnati College of Medicine, and Astha Malik, PhD, a post-doctoral research associate, and corresponding author Alexander Miethke, MD, director of the liver care center, were published Dec. 14, 2022, in Science Translational Medicine.
“Currently, there is no cure for sclerosing cholangiopathies such as biliary atresia or primary sclerosing cholangitis (PSC) outside of liver transplant,” Miethke says. “But as we learn more about the mechanisms behind disease progression, there may be ways to limit the organ damage and delay or even eliminate the need for a liver transplant.”
Is it the disease or the body’s response?
Scientists have known for years that bile duct damage has a cascading effect. If the ducts are malformed at birth or damaged later, bile acids that cannot be pushed out of the liver begin damaging the organ. This includes an immune response that inflames and damages remaining tissues, making the malfunctions even worse. Ultimately, liver function fails completely.
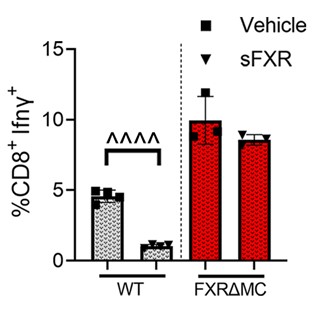
In the new study, the researchers show that this immune response, and not just the direct toxicity of the bile acids, contributes in powerful ways to the organ damage. They also demonstrate, in mouse models, that the response can be controlled.
Specifically, the team found that macrophage-related damage can be limited by activating the farnesoid X receptor (FXR) within macrophage cells. When FXR is activated in these cells, it can slow down production of inflammatory cytokines and constrain unwanted T cell multiplication. The protective effects of systemic FXR agonist therapies on liver and bile duct damage were shown in two different mouse models of sclerosing cholangitis and were validated with immune cells isolated from livers and blood of patients with biliary atresia or PSC.
“FXR activation in liver macrophages helps slow disease progression, which could prevent the life-threatening complications of biliary fibrosis and the need for organ transplantation,” Miethke says.
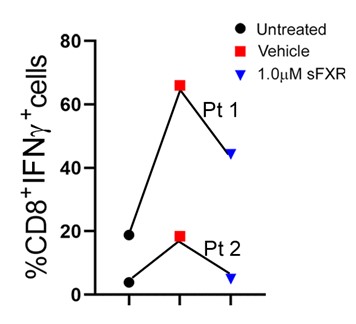
Next steps
It will require clinical trials in patients to determine whether FXR medications are safe for children with cholestatic liver diseases, and which one may be most efficacious. As this work progresses, Miethke says it will become important for studies of liver disease to take the inflammatory response into consideration.
“Suitable FXR agonists need to be systemically active, have specific chemical properties, and not activate FXR only in the intestine,” Miethke says. “Also, clinical endpoints in such studies should include immune markers assessing the effects of FXR therapies on macrophage function and T lymphocyte polarization, and not only measure the effects of these drugs on bile acid metabolism.”
About this study
Completing the many experiments needed to confirm these findings took several years. In addition to Shi, Malik and Miethke, Cincinnati Children’s co-authors included Annika Yang vom Hofe, BA, Celine Lages, PhD, Ruchi Singh, PhD, Kenneth Setchell, PhD, Louis Matuschek, BS, Mary Mullen, PhD, Ramesh Kudira, PhD, Wujuan Zhang, PhD, David Hildeman, PhD, and Chandrashekhar Pasare, PhD.
Funding sources for this study included the National Institutes of Health (R01DK095001-06, P30 DK078392), the Center for Autoimmune Liver Disease, and the Center for Translational Fibrosis Research.
View more discoveries from 50 research divisions and areas
Return to the 2023 Research Annual Report main features
| Original title: | Farnesoid X receptor antagonizes macrophagedependent licensing of effector T lymphocytes and progression of sclerosing cholangitis |
| Published in: | Science Translational Medicine |
| Publish date: | Dec. 14, 2022 |
Research By









