A New Era for Drug Safety Studies
Post Date: December 8, 2025 | Publish Date:
Cincinnati Children’s joins national ARPA-H effort to transform drug development with human organoids and AI
Of the thousands of promising medications that enter human clinical trials every year, more than 20% fail because traditional animal testing fails to predict safety issues.
That’s because animal models often cannot fully reflect the complexity of human biology nor how people differ from one another in their responses to treatment. As a result, some people experience harm during clinical trials and years of research and investment can be wasted in the process.
To address this challenge, scientists at Cincinnati Children’s are joining a national team on an up to $21M project called Computational ADME-Tox and Physiology Analysis for Safer Therapeutics (CATALYST). The program, funded by the Advanced Research Projects Agency for Health (ARPA-H), aims to revolutionize preclinical drug safety prediction by developing human-based models that accurately estimate toxicity and safety profiles for drug candidates.
The project is led by Inductive Bio, an AI drug discovery partner for biopharma that helps design and optimize higher quality drugs. The team will include Cincinnati Children’s experts Takanori Takebe, MD, PhD, Magdalena Kasendra, PhD, Tomoyuki Mizuno, PhD, and Bhagwat Prasad, PhD, and University of Cincinnati Bioengineering faculty member Riccardo Barrile, PhD. They will join colleagues from Inductive Bio, Amgen, Baylor College of Medicine, and Torch Bio (a spinout from University of Michigan College of Pharmacy).
Read the Inductive Bio announcement
The initiative, called DATAMAP (Digital Acceleration of Toxicity Assessment with Mechanistic and AI-driven Predictions), will integrate four technological advances: human organoids, microphysiological (“organ-on-chip”) systems, ex-vivo tissue and machine learning models. Together, these tools will allow researchers to predict drug toxicity earlier and more accurately than current methods.
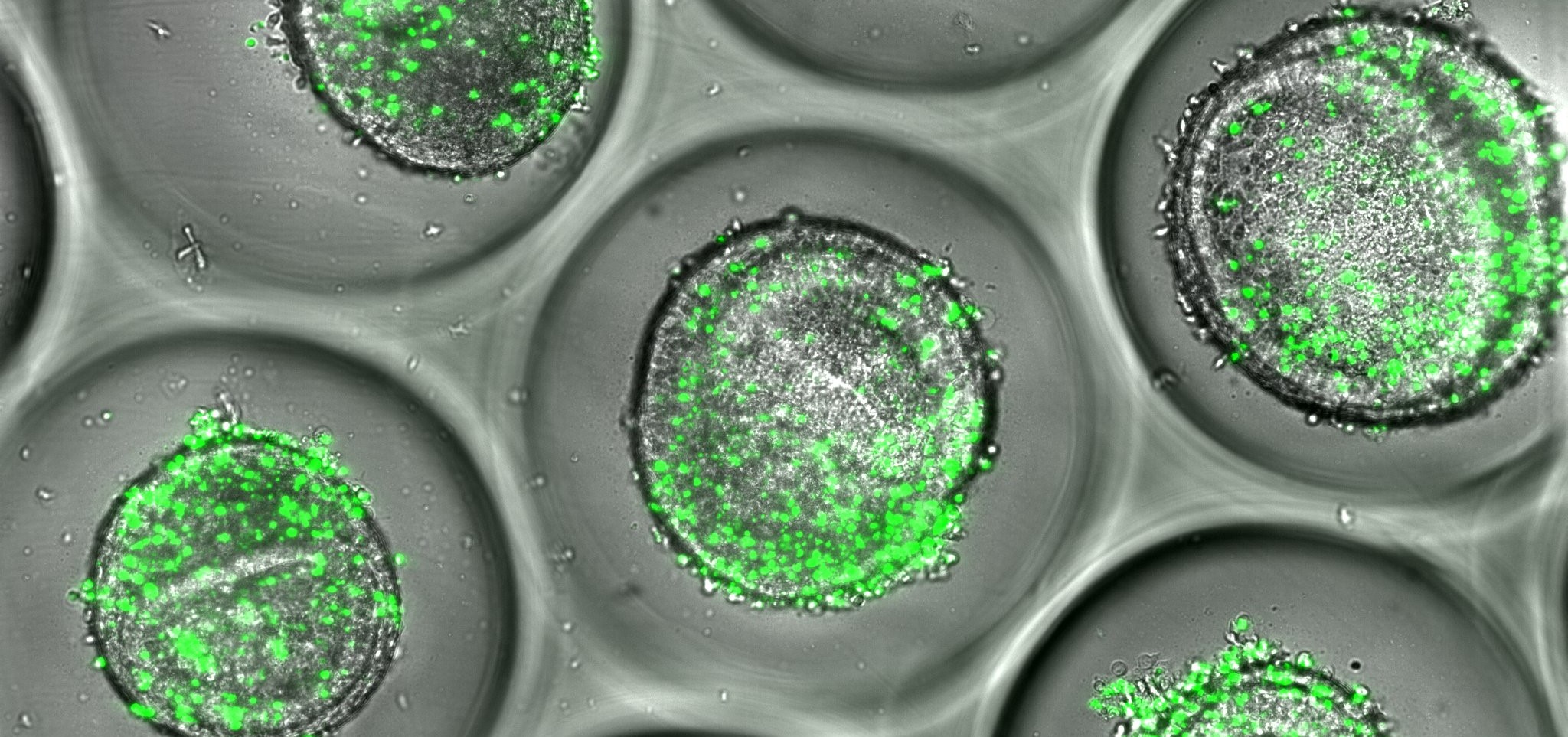
Organoids are tiny, three-dimensional tissues grown from human stem cells that behave like miniature organs such as the liver and heart. By generating organoids from people with different genetic and metabolic backgrounds, researchers can observe how potential medicines interact with diverse human biology.
Microphysiological systems will allow these organoids to be studied under controlled, reproducible conditions. The data generated will then be analyzed using machine learning to identify patterns, mechanisms, and risks that may not be visible through traditional testing.
“With human organoid data and AI, we are now entering an era where we can test drug safety directly in human-derived systems, allowing us not only to predict toxicity but to understand its mechanisms in real time. It’s a paradigm shift toward precision safety evaluation, one that could shorten development timelines, reduce costs, and make medicine more personal and humane,” says Takebe, who serves as director of commercial innovation at the Center for Stem Cell & Organoid Medicine (CuSTOM).
Cincinnati’s Children’s: A Leader in Organoid Science
Cincinnati Children’s has been at the forefront of organoid science for more than a decade. In 2010, institutional teams produced the first functional human intestinal organoids from induced pluripotent stem cells. In 2017, those pioneering efforts led to the creation of CuSTOM–a first-of-its-kind organoid research center uniting developmental biology, engineering, and clinical translation under one collaborative framework.
Within CuSTOM, scientists have developed a range of organoid platforms capable of modeling human development, disease and drug response. In particular, research led by the Takebe Lab has demonstrated the ability to customize highly functional liver organoids specifically suited for predicting drug-induced liver injury with unprecedented fidelity.
To ensure such breakthroughs can be deployed reliably and at scale, Cincinnati Children’s established the CuSTOM Accelerator, directed by Magdalena Kasendra, PhD, and supported in part by the Farmer Family Foundation. The Accelerator provides the automation, standardization, and operational rigor required to convert research innovations into reproducible platforms that meet the requirements of drug development and industry collaboration.
Together, the scientific innovation within CuSTOM and the translational pipeline of the CuSTOM Accelerator uniquely position Cincinnati Children’s to help define a new, human-centered model for drug development.
How DATAMAP Moves the Field Forward
Cincinnati Children’s researchers will generate diverse, patient-derived iPSC organoids that reflect real-world genetic and metabolic variation. These organoids will be integrated into microphysiological systems where controlled drug exposures will produce detailed molecular and functional datasets. These datasets will train DATAMAP’s machine learning frameworks, enabling predictions that connect biological signals to clinical outcomes and improve toxicity prediction.
The resulting “living, learning” dataset is intended to expand continuously as new donors, modalities, and disease contexts are added—widening the impact from safety de-risking to broader questions of efficacy, drug combinations, and matching the right therapy to the right patient
“What excites me most is that this is not a static model. As more donor lines, organ systems, and disease conditions are added, the system learns and improves. By bringing together academic innovators, industry partners, and AI experts, we are building a living, evolving resource that reflects real human diversity and will help guide safer, smarter medicines for years to come,” Kasendra says.
Looking Ahead
Work under DATAMAP will expand to larger and more diverse donor cohorts, additional organ systems, and more therapeutic modalities. The ultimate goal is a human-centered drug development framework that improves prediction, reduces late-stage failures, accelerates patient access to effective therapies, and reduces reliance on animal testing.
This effort is part of a broader vision at Cincinnati Children’s to translate organoid technologies into tools that benefit patients through safer, more effective, and more personalized treatments.
Learn more about CuSTOM) at:
https://www.cincinnatichildrens.org/research/divisions/c/custom
Don’t Miss a Post:
- Subscribe to the Research Horizons Newsletter
- Follow Cincinnati Children’s Research Foundation on Bluesky, X and LinkedIn