Vitamin A May Reduce Pancreatitis Risk During ALL Treatment
Research By: Mayur Sarangdhar, PhD | Maisam Abu-El-Haija, MD, MS | Anil Goud Jegga, DVM, MRes
Post Date: March 15, 2023 | Publish Date: March 15, 2023
Biomedical Informatics | Top Scientific Achievement

Consuming a diet rich in vitamin A or its analogs may help prevent children and young adults with acute lymphoblastic leukemia (ALL) reduce their risk of developing painful pancreas inflammation during chemotherapy treatment.
Details about this potential dietary solution to prevent a potentially life-threatening adverse event were published March 15, 2023, in Science Translational Medicine. The research team was led by Sohail Husain, MD, chief of Pediatric Gastroenterology, Hepatology, and Nutrition at Stanford University and Anil Goud Jegga, DVM, MRes, a computational biologist at Cincinnati Children’s.
For people with ALL, treatment with the enzyme asparaginase helps starve cancer cells by reducing the amount of asparagine circulating in the blood, which the cancer cells need but cannot make themselves. The medication, often used in combination with other chemotherapies, is given via injection into a vein, muscle, or under the skin.
However, an estimated 2% to 10% of asparaginase users develop inflammation of the pancreas in reaction to asparaginase treatment. For a third of these people, the symptoms can be severe.
Power of data mining
Jegga and colleagues developed predictive analytics using over 100 million data points encompassing gene expression data, small-molecule data, and electronic health records to understand more of the mechanisms driving asparaginase-associated pancreatitis (AAP) and identify potential interventions to prevent or mitigate AAP.
First, they analyzed massive amounts of gene expression data to reveal that gene activity associated with asparaginase or pancreatitis might be reversed by retinoids (vitamin A and its analogs). The team found more supporting evidence by “mining” millions of of electronic health records from the TriNetX database and the U.S. Federal Drug Administration Adverse Events Reporting System.
This number crunching and predictive analytics work included use of the AERSMine software developed at Cincinnati Children’s by Mayur Sarangdhar, PhD, MRes, and colleagues. The research team also studied data from mice experiments and compared plasma samples from people with ALL who developed pancreatitis and those who did not.
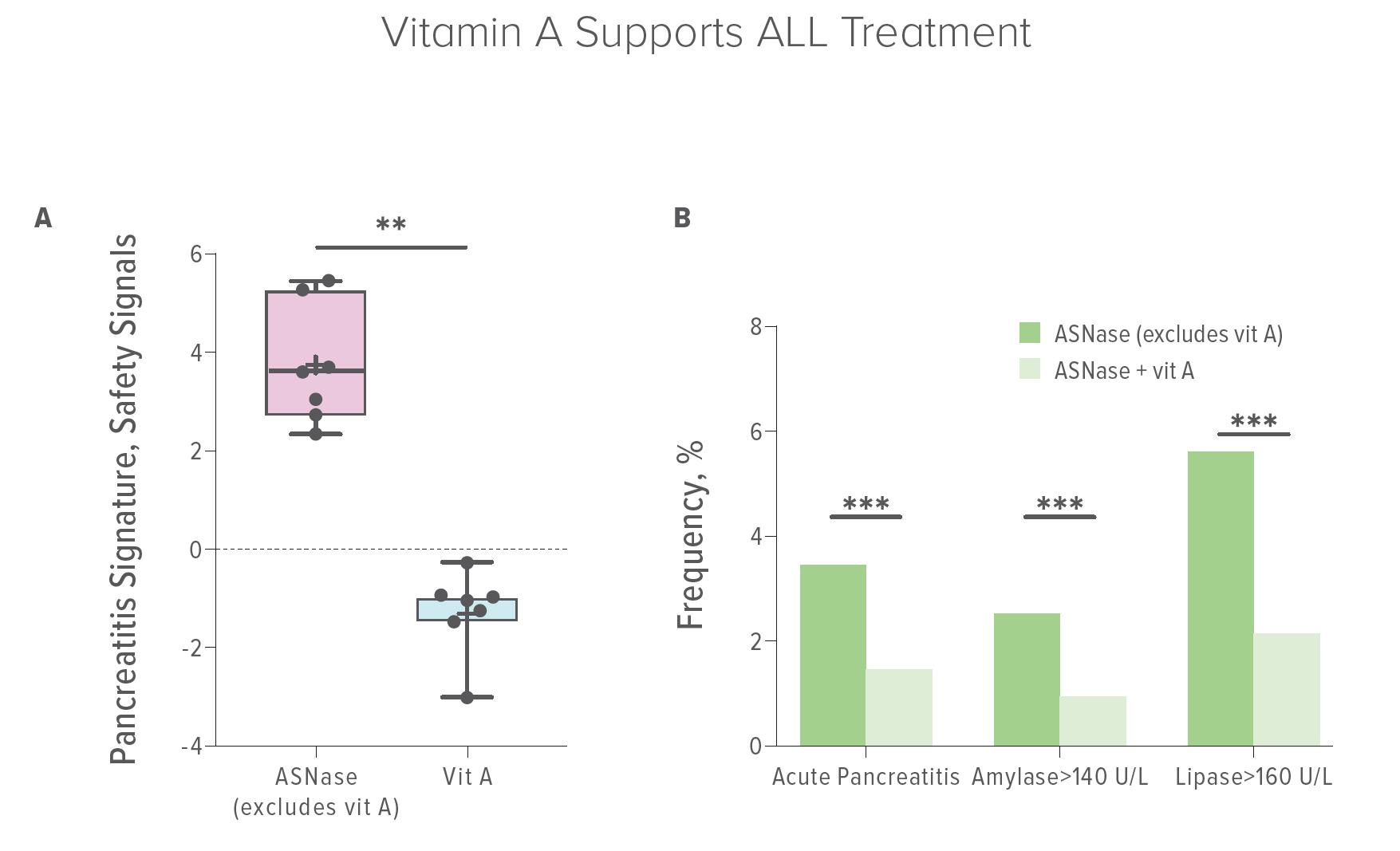
Ultimately, the team established two sets of human “real-world” experiences. They found that only 1.4% of patients treated with asparaginase developed pancreatitis when they were also taking vitamin A in contrast to 3.4% of patients who did not. Concomitant use of vitamin A correlated with a 60% reduction in the risk of AAP. Lower amounts of dietary vitamin A correlated with increased risk and severity of AAP.
“This study demonstrates the potential of mining ‘real-world’ data to identify therapy modifiers for improving patient outcomes. In cases where a primary drug induces toxicity but is critical to therapy, such as asparaginase, therapy modifiers, such as vitamin A and its analogs, may be of immediate relevance to patients on asparaginase and ‘at-risk’ for AAP,” says Sarangdhar, a co-first author of the study.
Says Jegga: “Our study highlights the power of heterogeneous data integration and analysis in translational research. By leveraging existing ‘omics and patient-centric data and a systems approach, we were able to identify new insights into the development of AAP and potential interventions to prevent or mitigate this side effect.”
Next steps
In some ways, learnings from this study could be applied immediately to patient care. However, more clinical research is needed to establish how much vitamin A would be needed to protect ALL patients from pancreatitis; and whether a protective level can be achieved by diet or via supplements. In fact, target vitamin levels may need to vary according to individual differences in metabolism.
More 2023 Research Highlights
Chosen by the Division of Biomedical Informatics
Wissel BD, Greiner HM, Glauser TA, et al. Automated, machine learning-based alerts increase epilepsy surgery referrals: A randomized controlled trial. Epilepsia. 2023;64(7):1791-1799. doi:10.1111/epi.17629
Pilarczyk M, Fazel-Najafabadi M, Kouril M, et al. Connecting omics signatures and revealing biological mechanisms with iLINCS. Nat Commun. 2022;13(1):4678. Published 2022 Aug 9. doi:10.1038/s41467-022-32205-3
Yang Z, Shikany A, Ni Y, Zhang G, Weaver KN, Chen J. Using deep learning and electronic health records to detect Noonan syndrome in pediatric patients. Genet Med. 2022;24(11):2329-2337. doi:10.1016/j.gim.2022.08.002
Andorf, Sandra et al. Estimating the Probability of Peanut Allergy Development in the Avoidance Arm of LEAP using Classification and Regression Tree Analysis. The Journal of Allergy and Clinical Immunology. 2023;151 (2): supplement, AB173. doi:10.1016/j.jaci.2022.12.543
Cazares TA, Rizvi FW, Iyer B, et al. maxATAC: Genome-scale transcription-factor binding prediction from ATAC-seq with deep neural networks. PLoS Comput Biol. 2023;19(1):e1010863. Published 2023 Jan 31. doi:10.1371/journal.pcbi.1010863
View more discoveries from 50 research divisions and areas
Return to the 2023 Research Annual Report main features
| Original title: | A systems approach points to a therapeutic role for retinoids in asparaginase-associated pancreatitis |
| Published in: | Science Translational Medicine |
| Publish date: | March 15, 2023 |
Research By

My research focuses on integrating high-dimensional computational approaches with systems biology knowledgebase to accelerate the discovery of novel drug-toxicity relationships buried in heterogeneous big data.

In my research, I am focused on acute pancreatitis, predictive models for severe pancreatitis and diabetes that occurs after acute pancreatitis. I’m also actively working on research in acute recurrent chronic pancreatitis and total pancreatectomy islet auto-transplantation arenas.

The mission of the Jegga Lab is to design, develop and apply novel and robust data-driven computational approaches that will accelerate the diffusion of genomics and biomedical data into translational research and education.