Minor Cell Population Plays Major Role in Triggering a Silent Subset of Inherited MDS Cases
Research By: Timothy Chlon, PhD | Daniel Starczynowski, PhD
Post Date: September 1, 2021 | Publish Date: Sept. 1, 2021
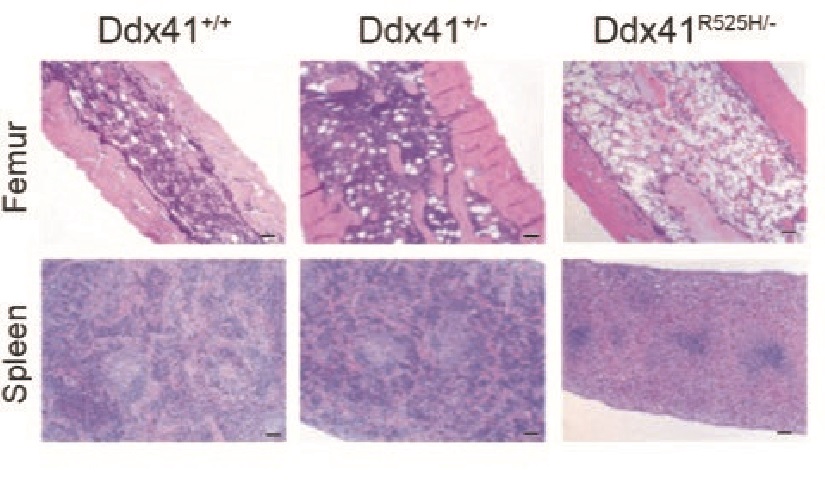
In mice, mutations in the DDX41 gene create a polluted environment where diseased blood stem cells can thrive
In the United States, as many as 187,000 people are coping with myelodysplastic syndromes (MDS) that damage the bone marrow’s ability to produce healthy blood cells.
In most cases, MDS is caused by mutations that arise in blood stem cells during a person’s life. These patients experience anemia, fatigue, and other complications, with a typical age of MDS diagnosis at 70 years and beyond. However, about 2% to 5% of people with MDS are born with mutations that predispose them to the condition, yet these people do not experience symptoms until their 60s.
In 2016, other scientists documented that people with this inherited form of adult MDS share mutations in the DDX41 gene, but it had not been clear what role the mutations played. Now, a study led by experts at Cincinnati Children’s Cancer and Blood Diseases Institute, published Sept. 1, 2021, in Cell Stem Cell explains the significance.
Their discovery was based on extensive work to develop a more accurate mouse model of human MDS caused by mutations in DDX41. An important facet of the disease seems to depend on cells that acquire an additional mutation in their other copy of the DDX41 gene, creating blood stem cells with two DDX41 mutations.
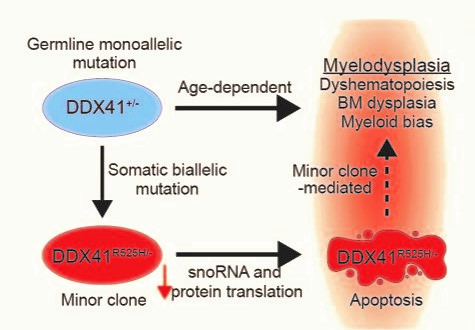
The team reasoned that these cells, which are relatively rare in the patient’s bone marrow, could indirectly affect the rest of the bone marrow and trigger abnormal blood production. In this way, this minor cell population could become a driver of MDS. Their findings suggest that targeting this minor cell population could lead to a treatment to prevent some cases of MDS.
“Basically, these rare cells help create a polluted bone marrow environment that in turn allows other stem cells with MDS-related gene mutations to thrive,” says Daniel Starczynowski, PhD, senior author of the study. “Without the presence of these trigger cells, the bone marrow might go on making blood cells normally as it had throughout the patient’s life.”
While this study focuses on MDS, similar types of crosstalk between cells with different combinations of mutations may play a role in other diseases.
“To our knowledge, this is the first evidence that inherited or de novo forms of MDS can be mediated by a disease-modifying minor clone in the bone marrow,” Starczynowski says.
What is MDS?
Myelodysplastic syndromes include a group of disorders that damage bone marrow function. About 75% of cases occur in people over age 60, but the condition also affects children and young adults. About one in three cases of MDS progresses to acute myeloid leukemia (AML), according to the MDS Foundation.
Sometimes MDS occurs as a side effect of radiation or chemotherapies for cancer. Some cases might be connected to toxic environmental exposures or to rare inherited diseases like Fanconi anemia. However, in most cases, the cause of MDS remains unknown.
Advanced cases of MDS require bone marrow transplantation. The average survival rate without a bone marrow transplant is about six years, but some patients can die within months from rapid bone marrow failure.
Detecting a silent trigger
Starczynowski and his colleagues have been studying MDS for years. After learning from other studies in the field that an inherited mutation in the DDX41 gene had been associated with MDS, the team set out to develop the mouse model in hopes of understanding more about the gene mutation.
“The cells with this additional DDX41 mutation do not comprise a large portion of the bone marrow. In fact, it’s hard to get cells with that combination of mutations to grow at all,” says Tim Chlon, PhD, first author on the study. “Once we realized that the cells were rare in the patient bone marrow, we thought that crosstalk occurring between these cells and the other cells in the bone marrow might play a role in disease onset.”
The mouse experiments reveal that blood stem cells with mutations in both copies of the DDX41 gene have defective machinery (called ribosomes) for making new proteins. This prevents the stem cells from multiplying and forming normal amounts of new blood cells. Since these cells do not multiply efficiently, they do not cause bone marrow failure by themselves, but they influence the other cells in the bone marrow and contribute to ineffective blood cell production.
This process may help explain why this subset of people with MDS can feel totally normal into late adulthood before the condition suddenly sets in.
Next Steps
The research team plans further studies to determine whether targeting cells with the minor clonal DDX41 mutation will change the course of MDS in mouse models, which if successful, could lead to potential human drug development.
The findings may also serve as a springboard for other research to look for similar crosstalk between potentially overlooked minor cell populations and their more abundant henchmen.
About the study
This work was supported by several grants from the National Institutes of Health (R35HL135787, R01DK102759, R01DK113639, K01DK121733, F32HL143993, R01HL132071, R35HL135795, and F31HL131140); Cancer Free Kids, the American Society of Hematology, the Edward P. Evans Foundation and the Vera and Joseph Dresner Foundation.
Read More About Blood Cancer Research at Cincinnati Children’s
NIH, Cincinnati Children’s Scientists Develop Potential Strategy Against Leukemia Drug Resistance
TRAF6 Role Extends Beyond MDS to Affect Innate Immune Signaling
Bone Marrow ‘Map’ Opens Path to Organoid-like Blood Stem Cell Production
Gene Targeting Reveals Therapy To Help Defeat mTOR Drug Resistance in AML
Small Molecule IODVA1 Shows Early Promise Against RAS-Driven Cancers
| Original title: | Germline DDX41 mutations cause ineffective hematopoiesis and myelodysplasia |
| Published in: | Cell Stem Cell |
| Publish date: | Sept. 1, 2021 |
Research By