Small Molecule IODVA1 Shows Early Promise Against RAS-Driven Cancers
Research By: Nicholas Nassar, PhD
Post Date: March 16, 2020 | Publish Date: March 12, 2020
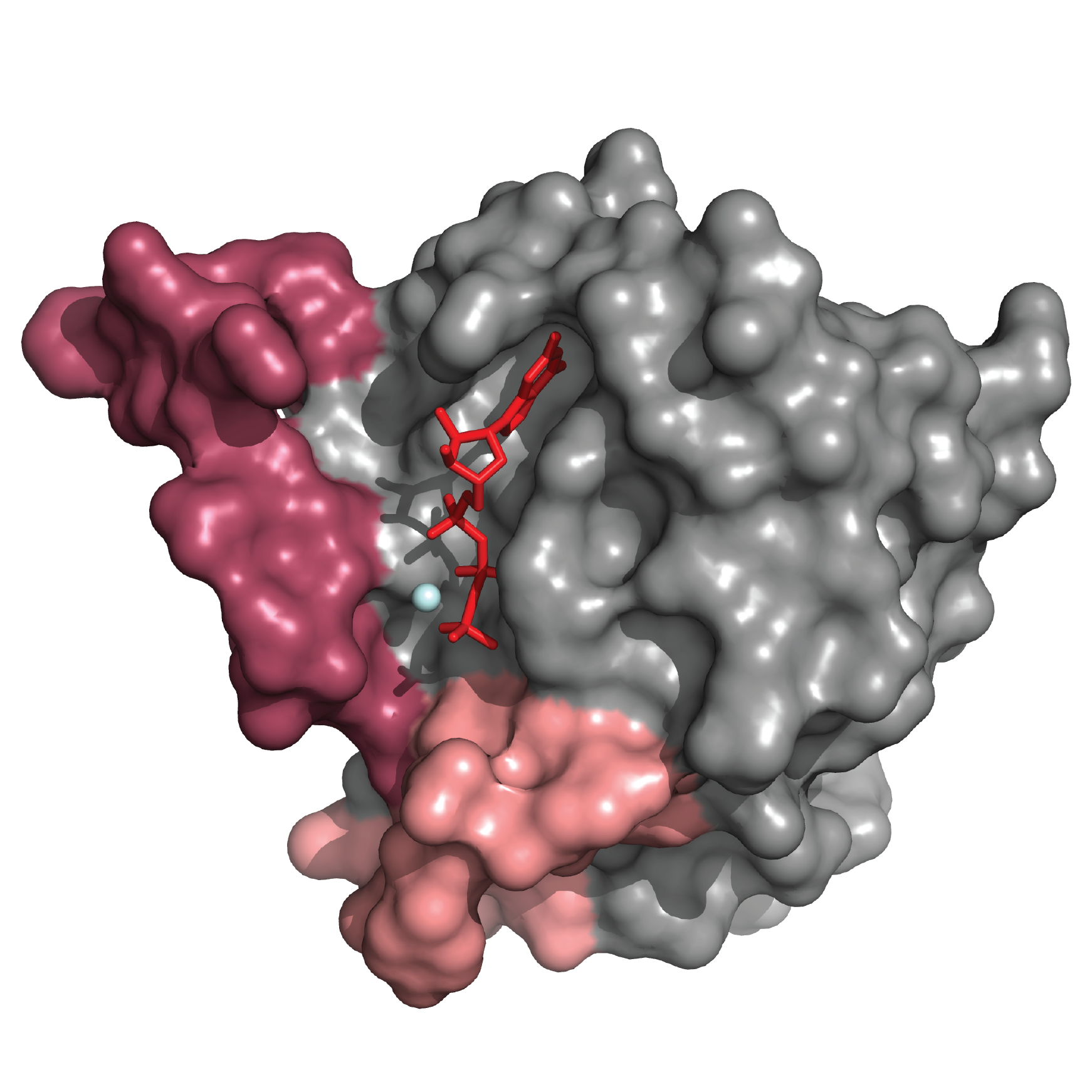
Cincinnati Children’s and University of Cincinnati team drills into an under-studied compound to find a key active ingredient that appears to inhibit oncogenic RAS signaling.
Cancer researchers have devoted decades to studying the multiple roles that Ras proteins play in tumor development.
When functioning correctly, these proteins control cell growth, gene expression, cell survival, and more. When Ras functions are disrupted, cancer often follows.
Ras gene mutations and related molecular pathway disruptions occur in approximately one-third of all human cancers–especially in lung and pancreatic cancers. Mutated Ras proteins also are implicated in genetic diseases called RASopathies.
“It is very hard to treat Ras tumors. Among targeted therapies, MEK and RAF inhibitors are used but the outcome is usually not good,” says Nicholas Nassar, PhD, a member of the Division of Experimental Hematology and Cancer Biology here and the principal investigator for the IODVA1 study published online March 12, 2020, in PLoS One.
High-Tech Treasure Hunt Produces Results
Looking for new and different Ras inhibitors started with a sophisticated search through a National Cancer Institute library of compounds with potential anti-cancer value.
The team accessed and sorted through a massive collection of 3D structural information in hopes of identifying a set of small molecules capable of binding to a specific and hard-to-reach target.
“We identified a NCI-compound that had everything we were looking for: good efficacy in 2D and 3D cultures, low cell toxicity, etc. Everything except one property: it was not a Ras inhibitor,” Nassar says.
Further study revealed that the NCI’s compound actually was a mixture of multiple compounds with unknown structures.
“Instead of dropping the NCI compound, we decided to go after the active ingredient in the mixture,” Nassar says.
This prompted the Cincinnati Children’s investigators to team up with experts in chemical synthesis at the University of Cincinnati.
“Two long years later, we identified the active ingredient that we called IODVA1,” Nassar says. “That kind of isolation doesn’t happen often especially at research hospitals.”
The Cincinnati Children’s team included Anjelika Gasilina, contributors Jacek Biesiada, PhD, Shailaja Hegde, PhD, David Milewski, PhD, Gang Ma, Tanya Kalin, PhD, Jarek Meller, PhD, William Seibel, PhD, Lisa Privette Vinnedge, PhD, and José Cancelas, MD, PhD. University of Cincinnati co-authors included: Gurdat Premnauth, Purujit Gurjar and Edward Merino, PhD.
With an exciting prospect in hand–now under patent application–the team began learning more about how IODVA1 functions.
Proof-of-Concept, More Work Ahead
IODVA1 targets a different arm of oncogenic RAS signaling than existing FDA-approved BRAF and MEK inhibitors already in clinical use. While existing drugs target the MAPK arm, IODVA1 targets the Rac arm.
“Much more work needs to be done to determine if this compound, or a more potent analog, can go into clinical trials. Long-term, we think a combination of targeting both arms of Ras signaling will do the most to improve cancer patient outcomes,” Nassar says.
The PLOS One paper represents “Part One” of the IODVA1 story. More details about the exact mechanism of function for the compound have been submitted for publication to another journal.
“Now that we understand at the molecular level how IODVA1 works, we are testing it on patient-derived models of leukemia, especially models of resistance to current therapies because this is where the value of IODVA1 is,” Nassar says. “Later, we will branch to solid tumors. Breast cancer is on our radar.”
| Original title: | IODVA1, a guanidinobenzimidazole derivative, targets Rac activity and Ras-driven cancer models |
| Published in: | PLOS One |
| Publish date: | March 12, 2020 |
Research By