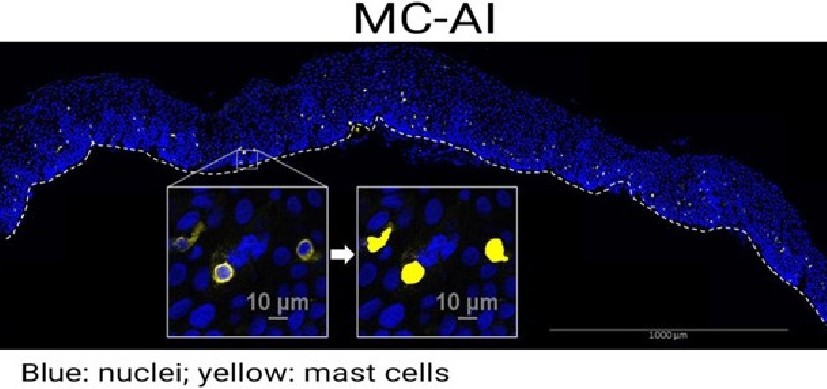
Biological Agent Successfully Treats Eosinophilic Esophagitis
Research By: Margaret Collins, MD | Marc Rothenberg, MD, PhD
Post Date: December 22, 2022 | Publish Date: Dec. 21, 2022
Pathology | Top Scientific Achievement


Benefits of weekly doses of dupilumab detailed in The New England Journal of Medicine
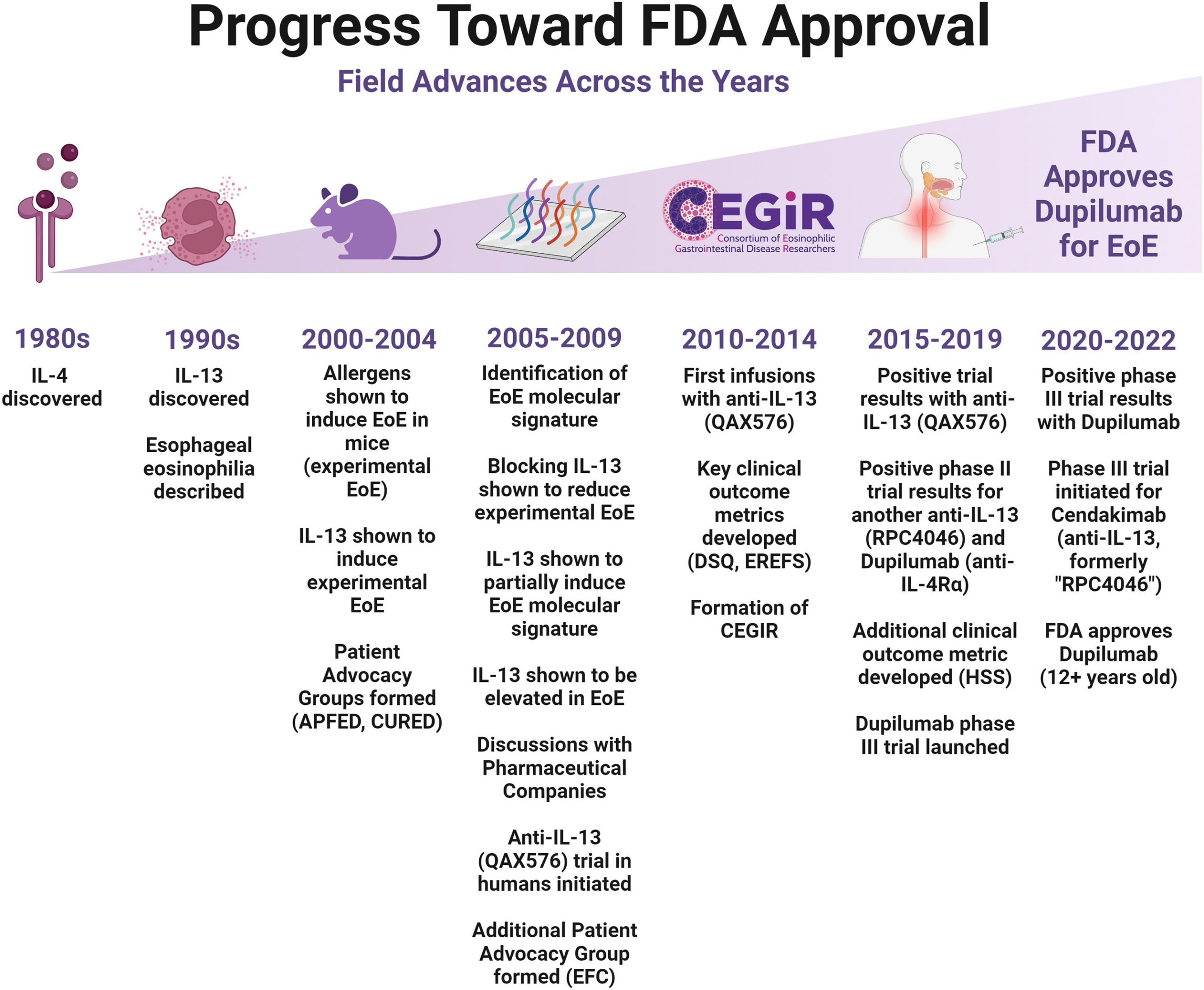
In May 2022, the FDA approved the first drug for eosinophilic esophagitis (EoE), a severe allergic disease in which the body treats the majority of foods as a harmful invader. This drug, dupilumab, blocks the signaling of key immune proteins, interleukins 4 and 13 (IL-4 and IL-13), which have essential roles in EoE.
Scientists at Cincinnati Children’s took part in an international clinical trial of dupilumab in adolescents and adults with EoE. The findings—published Dec. 21, 2022, in The New England Journal of Medicine—are the first to demonstrate that dupilumab is effective in treating EoE.
Histology (examination of microanatomy) and symptoms improved when dupilumab was given once a week, which is now the FDA-approved dosing regimen for dupilumab for EoE.
AN ONGOING TRIAL
“These findings from this three-part clinical trial are already benefiting patients and clinical practice,” says co-first author Marc Rothenberg, MD, PhD, director of the Division of Allergy and Immunology and the Center for Eosinophilic Diseases at Cincinnati Children’s and principal investigator of the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). “I am pleased with the strongly positive data that this trial yielded, supporting the use of this FDA-approved therapeutic while we continue researching even better treatments and a cure for eosinophilic esophagitis.”
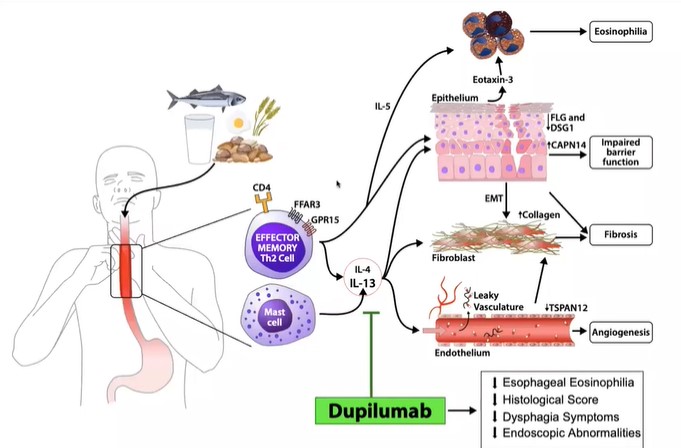
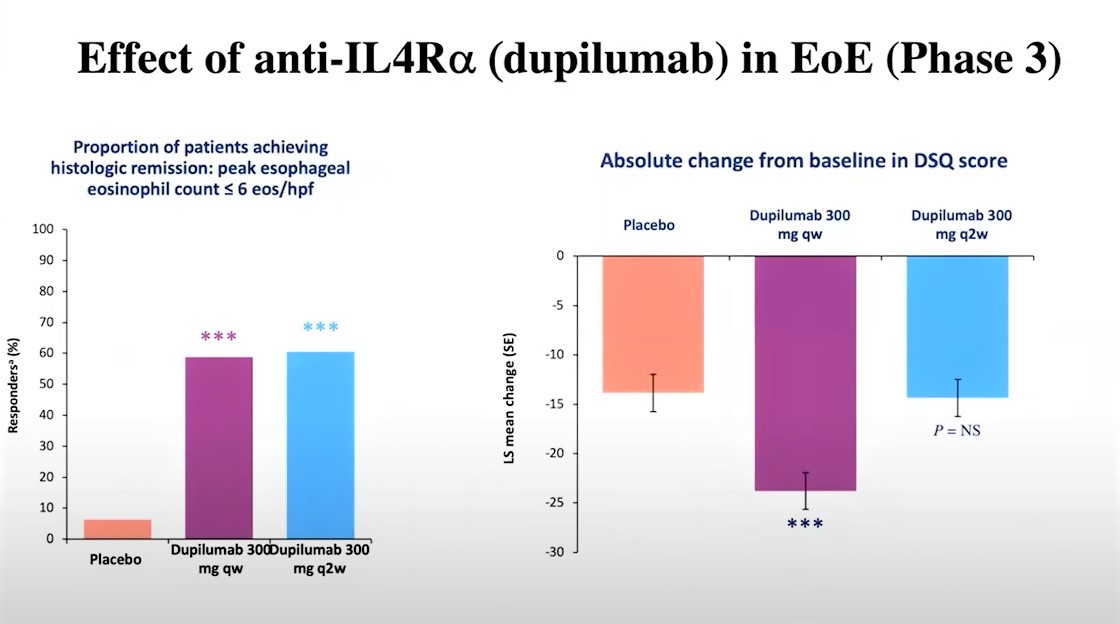
For the first part of this phase III clinical trial, adolescents and adults aged 12 years and older with EoE were randomized to 24 weeks of weekly subcutaneous dupilumab (300 mg) or placebo. Results from this first part were promising, as histologic remission was achieved in 59.5% for dupilumab versus 5.1% for placebo.
For the second part of this trial, adolescents and adults with EoE were randomized to 24 weeks of weekly dupilumab (300 mg), biweekly dupilumab (300 mg), or placebo. Histologic remission was achieved in:
- 58.8% of patients who were administered dupilumab on a weekly basis
- 60.5% of patients who were administered dupilumab biweekly
- 6.3% of patients who were administered a placebo.
However, patient symptoms did not improve by the end of the 24 weeks with biweekly dupilumab despite the histologic signs of the disease improving.
For the third part of the study, patients who continued to receive weekly dosing of dupilumab for an additional 24 weeks showed sustained improvements.
“This study leveraged two decades of translational science from my research laboratory, including findings supporting EoE being mediated by type 2 immune responses and the involvement of interleukins, including interleukin 13,” says Rothenberg. “We’re grateful to the academic researchers, industry partners, and patient and family collaborators, who have all made this work possible.”
Looking ahead, the results of this trial will continue to improve how dupilumab is used in clinical practice, ultimately affecting better outcomes for children and adults with EoE.
ABOUT THE STUDY
In addition to Rothenberg, Cincinnati Children’s co-authors included Margaret Collins, MD.
This clinical trial was funded by Sanofi and Regeneron Pharmaceuticals, Inc. (ClinicalTrials.gov number, NCT03633617)
Learn More
Read more about EoE research at the Rothenberg CURED Lab
Read more about the FDA approval of Dupilumab for EoE
More 2023 Research Highlights
Chosen by the Division of Pathology
Puneet Agarwal, Avery Sampson, Kathleen Hueneman, Kwangmin Choi, Xueheng Zhao, Jessica Galloway-Pena, Kenneth Setchell, Daniel T. Starczynowski; A Circulating Microbial Metabolite Drives the Clonal Expansion of Pre-Leukemic Cells. Blood 2022; 140 (Supplement 1): 975–976. doi: https://doi.org/10.1182/blood-2022-159124
Sumazin P, Peters TL, Sarabia SF, et al. Hepatoblastomas with carcinoma features represent a biological spectrum of aggressive neoplasms in children and young adults. J Hepatol. 2022;77(4):1026-1037. doi:10.1016/j.jhep.2022.04.035
Bouffi C, Wikenheiser-Brokamp KA, Chaturvedi P, et al. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nat Biotechnol. 2023;41(6):824-831. doi:10.1038/s41587-022-01558-x
Wu LMN, Zhang F, Rao R, et al. Single-cell multiomics identifies clinically relevant mesenchymal stem-like cells and key regulators for MPNST malignancy. Sci Adv. 2022;8(44):eabo5442. doi:10.1126/sciadv.abo5442
View more discoveries from 50 research divisions and areas
Return to the 2023 Research Annual Report main features
| Original title: | Dupilumab in Adult and Adolescent Patients With Eosinophilic Esophagitis |
| Published in: | The New England Journal of Medicine |
| Publish date: | Dec. 21, 2022 |
Research By


The Rothenberg CURED Research Laboratory, supported by the Campaign Urging Research for Eosinophilic Diseases (CURED), is focused on elucidating the mechanisms of allergic responses, especially in mucosal tissues such as the gastrointestinal tract and lung.