Preclinical Science: Building the Platforms of Discovery
Post Date: January 24, 2024 | Publish Date:

Before the next breakthrough treatment can be evaluated in clinical trials, innovative ideas require years of preclinical testing. Experts at Cincinnati Children’s not only carry out such work, they also help invent and improve the testing methods, tools and platforms. These are some of the many ways our lab teams work behind the scenes to bridge the gap between basic science discoveries and improved medical care.
From developing advanced organoids to inventing ways to deliver therapy to muscle tissues, Cincinnati Children’s researchers are leaders in creating new tools that can accelerate the chase for cures

Next-Generation Organoids Right Now
Cincinnati Children’s has been a leader in organoid medicine since late 2010, when Jim Wells, PhD, and colleagues published a study in Nature detailing how they created the world’s first functional, three-dimensional intestinal organoid from induced pluripotent stem cells.
In the years since, the team formed the Center for Stem Cell & Organoid Medicine (CuSTOM), recruited even more outstanding scientists, and published a series of successes at developing mini versions of the stomach, esophagus, colon, liver, pancreas, kidney, lungs, and more. Here, experts are growing organoids to mimic blood vessel functions and brain organoids to explore neuron formation and more.
In fiscal 2023, researchers here published two especially noteworthy findings that may advance the field of regenerative medicine.
In January 2023, in Nature Biotechnology, first author Carine Bouffi, PhD, corresponding author Michael Helmrath, MD, led a team of more than 20 scientists from five research centers to report achieving a major milestone: creating the first “next-generation” intestine organoids that include resident immune cells.
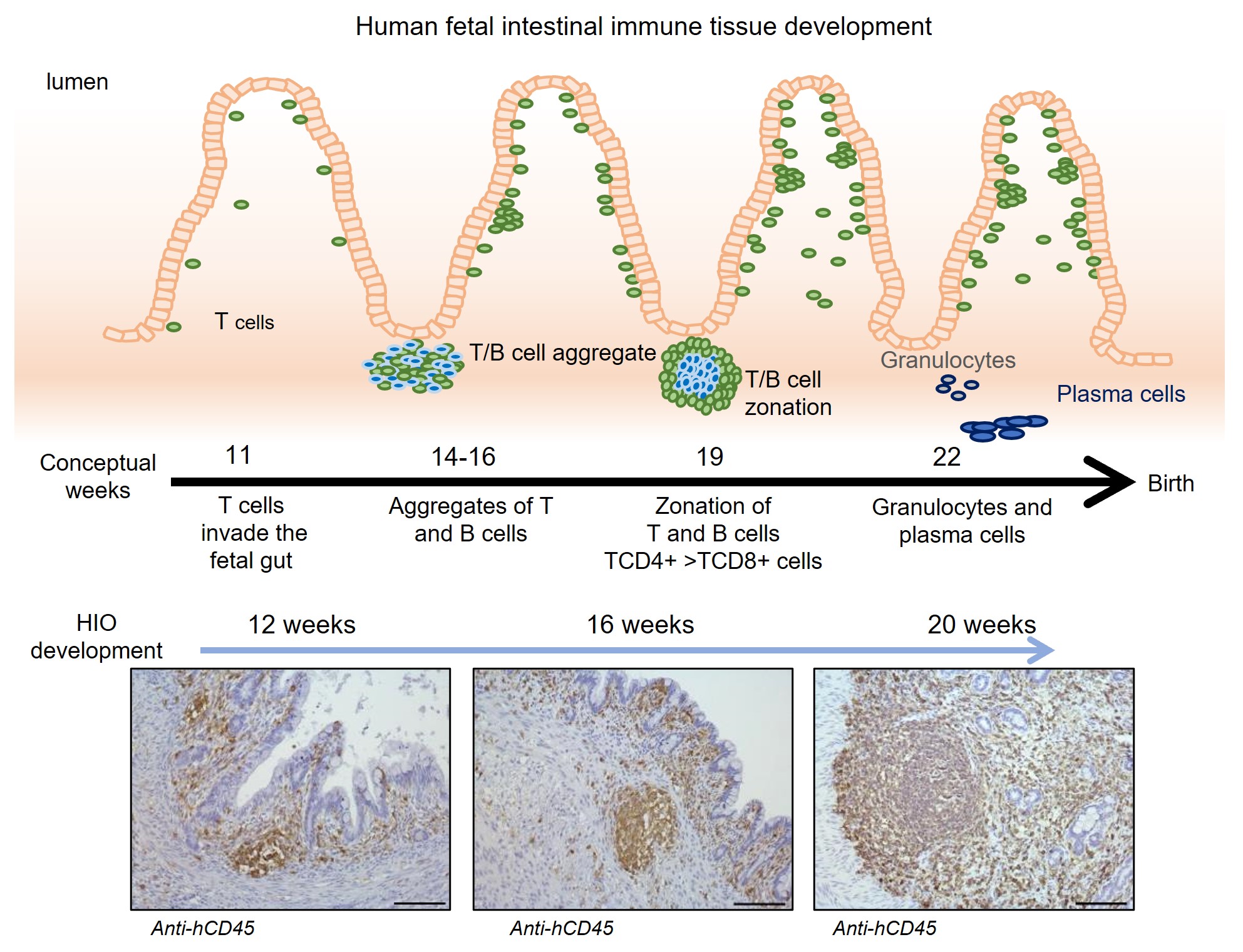
The co-authors say this is the first in vivo organoid of any type (heart, liver, stomach, etc.) to incorporate a functional immune system. This success at adding immune response capability–combined with previous success at layering in functional nerves–has inspired Cincinnati Children’s and other sponsors to invest millions to further prepare intestine organoid technology for potential human clinical trials.
“Not only does the organoid support the migrating immune tissues—rather than rejecting them—but those immune cells and structures go on to improve the development of the intestine itself, specifically its ability to recognize foreign antigens,” Helmrath says.
As organoids become more sophisticated, they are becoming more useful as tools to study disease and test potential treatments by using functional human tissue vs relying so heavily on animal models. And in November 2023, scientists here worked with colleagues at the Medical University of South Carolina and other centers to develop a next-generation colon organoid that also has immune cells built into its tissues.
A Humanized Platform for Obesity Research
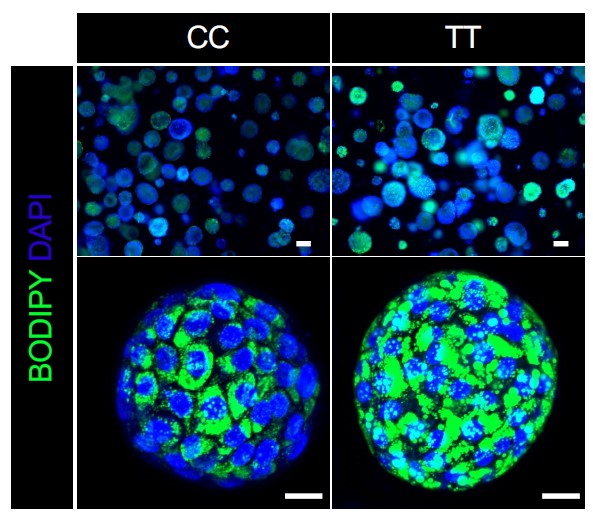
The long-predicted use of human organoids as customizable testing platforms is beginning to take off. In October 2022, in Cell, a multinational team led by Takanori Takebe, MD, PhD, developed the first liver organoid that reflects damage caused by nonalcoholic steatohepatitis (NASH), an obesity-driven condition that goes beyond fatty liver to cause life-threatening liver inflammation and scarring. NASH also can trigger liver cancer.


The novel approach used by Takebe and colleagues revealed genetic clues that could not have been found using animal models. The discovery suggests that specific changes in clinical care may be needed to help people with an all-too-common combination of diabetes and fatty liver disease.
Organoid development at Cincinnati Children’s actually spans multiple parts of the research spectrum. Here, researchers have made several basic science breakthroughs just to produce organoids. They have started using organoids in many ways to better understand the mechanisms behind multiple diseases, and have begun using organoids in preclinical testing of promising therapies.
In the years ahead, leaders at CuSTOM predict that organoids may become a treatment modality in their own right as lab-grown tissues derived from individual patients are transplanted back to them to repair damaged and dysfunctional organs.
Learn how you can support CuSTOM’s mission
Introducing Viral Fusogen Therapy
Researchers at Cincinnati Children’s and many other centers worldwide have been racing to find safe ways to deliver gene-based therapies to re-program parts of the human body in hopes of reversing the effects of diseases with known genetic causes.


Many such diseases have been identified, but only some have reached clinical trials to evaluate potential gene therapies. So far, scientists have focused on using modified viruses as delivery vehicles to insert corrected genetic information into human cells.
Now, research from first author Sajedah Hindi, PhD, and corresponding author Doug Millay, PhD, both with the Division of Molecular Cardiovascular Biology, have reported early success at using a twist on the viral delivery approach.
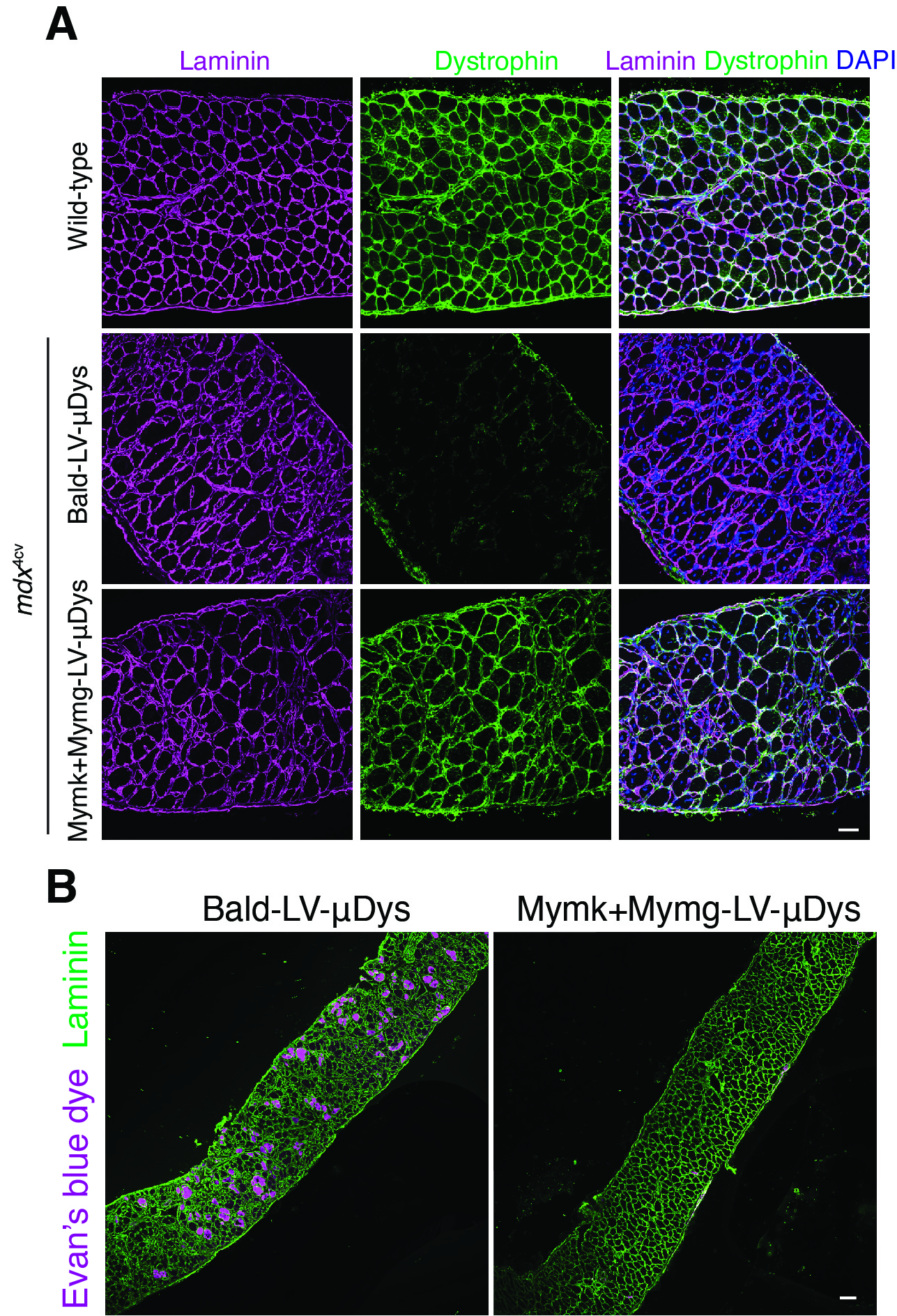
Their work, published April 18, 2023, in Cell, reveals that modified viruses, engineered with the fusogens Myomaker and Myomaker, can be used as vectors to deliver a functional version of a muscle formation gene that gets mutated in people with Duchenne muscular dystrophy (DMD).
Millay has been a leader in characterizing how Myomaker and Myomerger work. Until now, it was not known whether these proteins, which mainly function on cell surfaces, could bind with viruses. Now, his team’s work has translated into a preclinical mouse model that can be used to evaluate potential human treatment for DMD and perhaps beyond.
“This modified viral vector appears to be a promising tool for delivering a potential lifelong supply of the gene that is absent in people with DMD,” Millay says. “The unique advantages of this vector provide an opportunity to significantly impact gene therapy for a myriad of muscle diseases.”
In Rare XIAP-Deficiency, Quercetin Shows Promise Against Hyperinflammation
XIAP (X-linked inhibitor of apoptosis) deficiency is a rare inborn error of immunity that causes tissue-damaging hyperinflammatory disease. Currently, children with this condition often depend upon high-risk allogeneic hematopoietic cell transplantation.


However, in mouse models of the condition, quercetin—a natural flavonoid antioxidant sold in some stores—acted as a safe and effective inflammasome inhibitor, according to a study published in August 2022 in the journal Blood. The study was led by first authors Samuel Chiang, PhD, and Erika Owsley, BS, and corresponding author Rebecca Marsh, MD, of the Division of Bone Marrow Transplantation and Immune Deficiency.
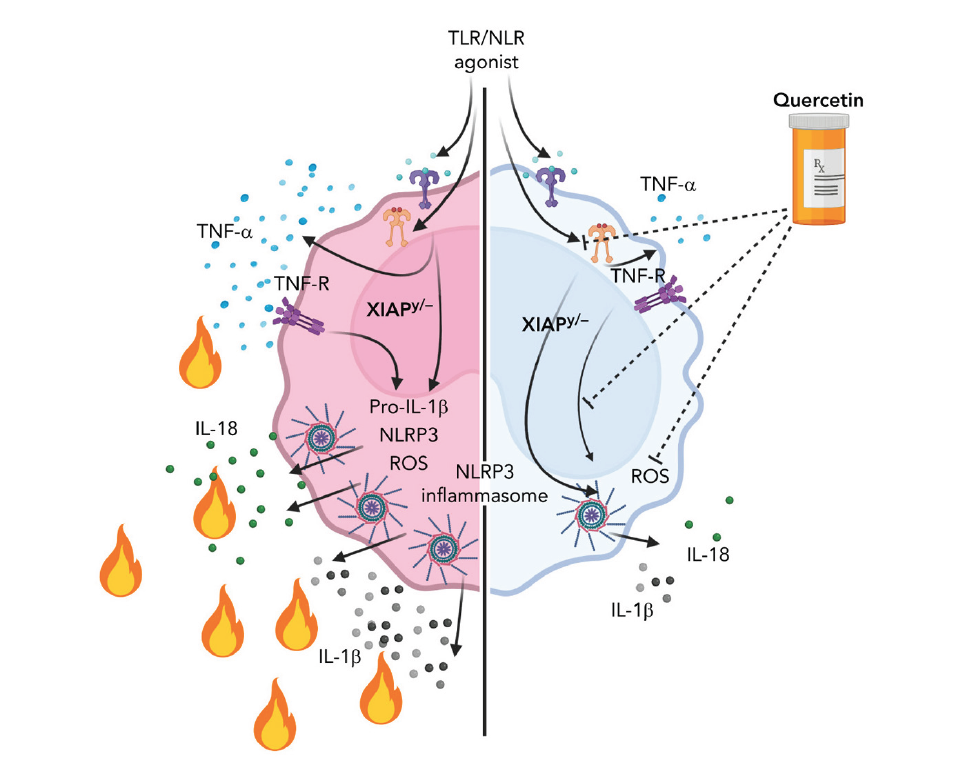
“Quercetin is an exceptionally promising agent given that it is a naturally occurring ROS scavenger, and it profoundly inhibits NLRP3 inflammasome priming. It also inhibited TNF-α secretion, which is beneficial in the setting of XIAP deficiency,” Marsh says.
The co-authors say work has begun to advance this therapy to human clinical trials.
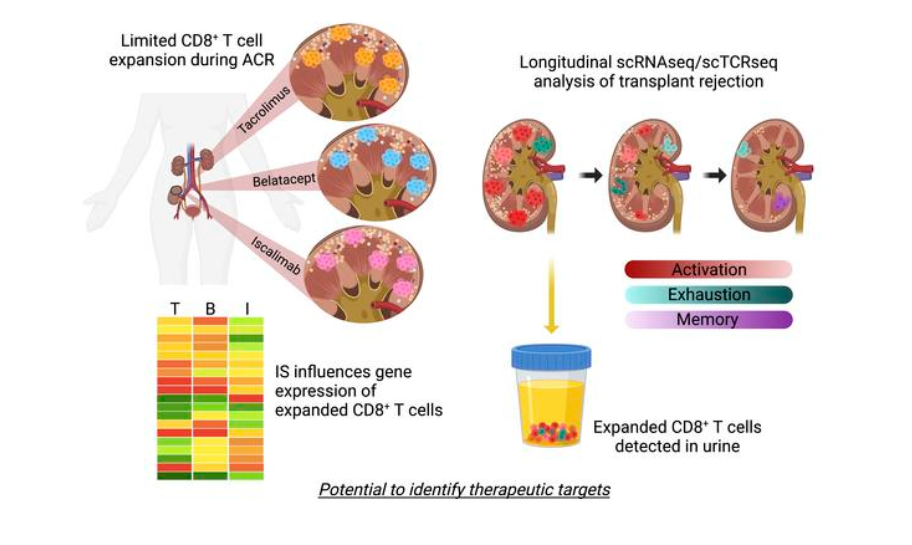
Can a urine test predict kidney rejection?
It took eight years of teamwork, but research led by experts at Cincinnati Children’s and the University of Cincinnati may help break a decades-long logjam in treating kidney transplant organ rejection.


In findings detailed May 25, 2023, in JCI The Journal of Clinical Investigation, scientists conducted a far-reaching set of single-cell analyses to identify the most specific cellular signatures to date for kidney transplant rejection. The findings reveal new potential targets for drug development that could advance the field. Importantly, the rare immune cell type that helps drive harmful inflammation during kidney rejection also shows up in urine, which suggests a potential alternative to collecting tissue biopsies to monitor organ health post-transplant.
The study was led by first author Tiffany Shi, an MD/PhD student, and senior co-authors David Hildeman, PhD, Division of Immunobiology, and E. Steve Woodle, MD, professor of surgery with the UC College of Medicine.
“The available treatments for stopping a rejection event have not changed much in decades. These cellular signatures open the door to establishing an entire new set of anti-rejection therapies,” Hildeman says.