Novel CAR NK-Cell Technology Could Lead to New Treatments for Lupus, Other Incurable Diseases
Research By: Seth Reighard, PhD | Hermine Brunner, MD, MSc, MBA | Stephen Waggoner, PhD
Post Date: May 11, 2020 | Publish Date: April 21, 2020
The explosion in cellular immunotherapy that has revolutionized cancer care in recent years may soon begin showing potential application in treatment for lupus and other autoimmune diseases, thanks to a laboratory breakthrough led by experts at Cincinnati Children’s and published in the journal Cell Reports Medicine.
In cancer, CAR T-cell therapy involves engineering T cells with chimeric antigen receptors (CAR) that allow them to recognize specific molecules on the surface of tumor cells. For certain forms of leukemia, some lung cancers, and other malignancies, this form of cellular immunotherapy has been life-changing for patients. Recent evidence suggests engineering natural killer (NK) cells to express CAR may be equally effective as T cells but with increased safety and clinical feasibility.
There is growing interest in the safety and efficacy of applying CAR cellular therapies to deadly and incurable autoimmune diseases. However, finding specific cell targets for diseases such as lupus has been much more difficult—until now.
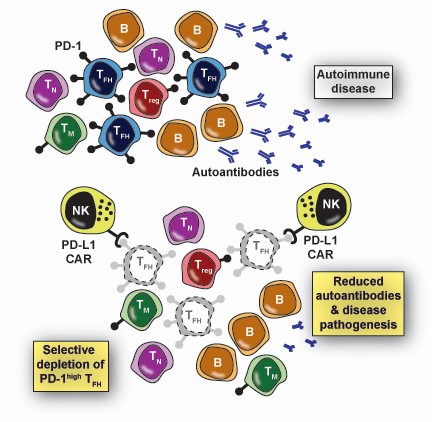
In a first-of-its-kind discovery, a team of Cincinnati Children’s scientists led by Seth Reighard, PhD, Stephen Waggoner, PhD, and Hermine Brunner, MD, MSc, MBA, has engineered a CAR with the potential to revolutionize care of patients with lupus. When expressed by human NK cells, this CAR enables targeted elimination of T follicular helper (Tfh) cells without harming other types of T-cells.
This treatment showed specificity in human cells in lab tests, and improved disease measures in a humanized mouse model of lupus—two key early signs of progress that suggest further research is warranted.
“This is the first method to specifically remove an otherwise intractable population of harmful cells,” Waggoner says. “We think targeting them will be safe and clinically beneficial in multiple diseases. Our approach started with lupus because the disease is a leading cause of death in young women for which a cure is presently lacking.”
The study appears in the first issue of the new, open access journal Cell Reports Medicine, which also carries a commentary about this new approach from Cecile King, PhD, an immunology expert at the Garvan Institute of Medical Research in Australia.
“Dysregulated Tfh cells are associated with the development and severity of several autoimmune diseases and T cell malignancies,” King states. “Indeed, the central role of Tfh cells in many diseases has made them a major target for therapeutic modulation. The study by Reighard et al. provides … exciting proof-of-principle evidence for the use of CAR NK cells in Tfh-driven diseases.”
How do CAR NK cells work?
The lupus-driving cells that the team wanted to eliminate lack cell-surface targets unique enough to distinguish them from other, desirable cells. To achieve selective targeting, the team realized a cardinal feature of Tfh cells that could be exploited by carefully engineering the biochemistry of the CAR molecule.
Specifically, Tfh cells express much greater quantities of a surface receptor, programmed cell death protein 1 (PD-1), than other cells that also express this receptor. Since activation of a CAR expressing NK cell is dependent on the strength of interaction between the CAR and its target receptor, as well as the number of such interactions between an NK cell and a target, the team engineered a CAR with relatively weak binding to PD-1. As a result, only cells like Tfh that exhibit high expression of PD-1 trigger activation of the CAR NK and are eliminated as a result, while cells with lower levels of PD-1, including regulatory T cell (Treg) and memory T cells, are spared.
These programmed killer cells show early signs of potential as a therapy for systemic lupus erythematosus (SLE), which affects 20-150 per 100,000 people in the U.S. In fact, lupus ranks in the five causes of death among African American and Hispanic women, aged 15-34.
In addition, aberrant Tfh responses play roles in several other autoimmune diseases, including Sjögren syndrome, juvenile dermatomyositis, multiple sclerosis, type 1 diabetes, and rheumatoid arthritis.
Although the potential toxicity of selectively eliminating Tfh remains unexplored, the preservation of naive and memory CD4 T cells as well as B cells and other types of immune cells suggests that the state of immunodeficiency induced by these CAR NK cells will be far less severe than other immunotherapeutic strategies applied to autoimmune disease (e.g., rituximab).
Discovery based on years of research
This advance in CAR technology build upon previous work by Waggoner and colleagues in 2015 and 2018 that revealed how NK cells play surprising regulatory roles in infection and autoimmune disease.
The conceptual connections between infections and autoimmune diseases were further strengthened by a discovery led by John Harley, MD, PhD, and colleagues at Cincinnati Children’s. In a 2018 study in Nature Genetics, they revealed how the Epstein-Barr virus uses groups of transcription factors to alter human DNA in ways that can increase a person’s risk of developing lupus, multiple sclerosis, type 1 diabetes, and other diseases.
What’s Next?
More work is needed to determine how much benefit can be gained by disrupting the role of Tfh cells in lupus and other conditions. Concerns to address also include how to prevent the killer cells from attacking non-targeted “good” cells, and how to efficiently deliver the therapeutic cells.
Researchers are working to develop “suicide switches” for CAR NK cells that would make them safer for clinical use, Waggoner says. But importantly, NK cells appear to pose lower toxicity risk than CAR T-cell therapies in multiple clinical trials in cancer patients. Given the contributions of T cells to disease pathogenesis in lupus and other autoimmune disease, therapeutic NK cells likely yield additional benefit in these contexts.
Although the present study was performed with a human NK-cell line approved for clinical use by the FDA, the team envisions flexibility in the clinical application of the new CAR to lupus. CAR engineering of patient cells or cells from unrelated donors, including cord blood or induced pluripotent stem cell-derived NK cells, have all demonstrated excellent safety profiles while maintaining desirable efficacy in clinical trials.
“The CAR can be introduced to various effector cells using micro RNA transfection, transposons, or viral vectors,” Waggoner says. “Freezers full of CAR-expressing induced pluripotent stem cell-derived NK cells would provide an off-the-shelf product that could be rapidly and repeatedly administered to numerous patients in order to quell harmful flares of disease activity and promote sustained disease remission.”
If successes continue, a clinical trial might be possible within the next few years, Waggoner says.
Co-authors of the paper also included: Stacey Cranert, PhD, Kelly Rangel, MS, Ayad Ali, BS, Ivayla Gyurova, MS, Arthur de la Cruz-Lynch, BS, Jasmine Tuazon, BS, Marat Khodoun, PhD, Leah Kottyan, PhD, and David Smith, MD, PhD.
| Original title: | Therapeutic Targeting of Follicular T Cells with Chimeric Antigen Receptor-Expressing Natural Killer Cells |
| Published in: | Cell Reports Medicine |
| Publish date: | April 21, 2020 |
Research By