Large-Scale Organoid Testing System Emerges For Predicting Risk of Liver Damage from New Medications
Research By: Takanori Takebe, MD
Post Date: October 8, 2020 | Publish Date: Oct. 8, 2020
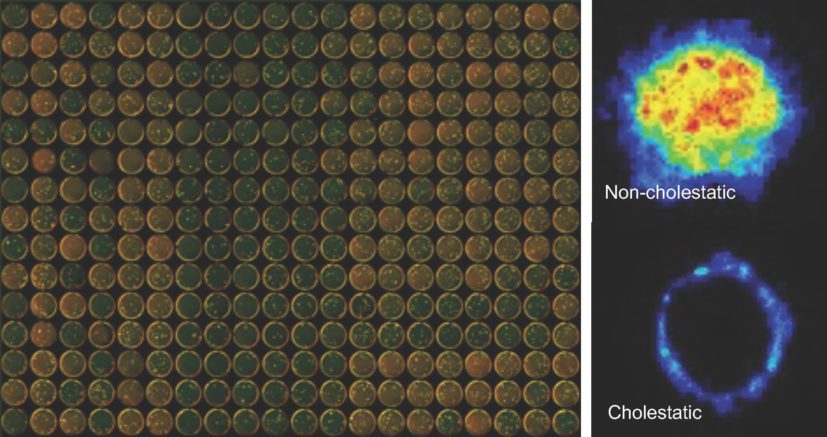
One major goal in the rapidly expanding world of organoid development has been to provide a way to test potential disease treatments in human testing models that do not require placing real people at risk.
Now, a study in the journal Gastroenterology, led by Takanori Takebe, MD, at the Cincinnati Children’s Center for Stem Cell and Organoid Medicine (CuSTOM), reports success at developing a human liver-in-a-dish testing platform that will allow pharmaceutical companies to assess whether an experimental medication will cause liver injury before giving the drug to human subjects in clinical trials.
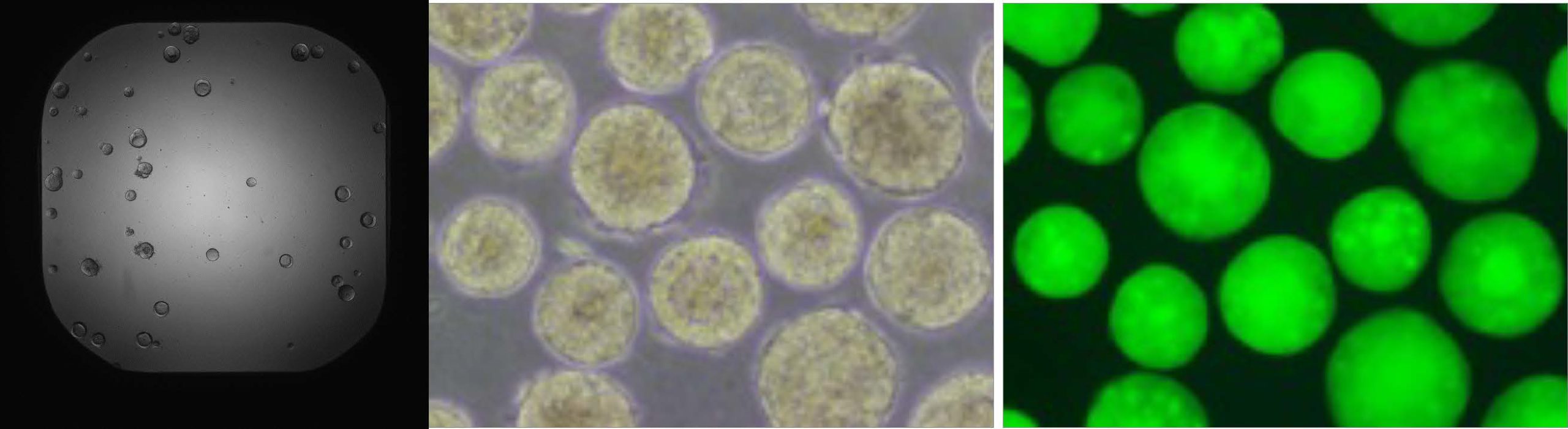
The new study takes a major step forward in transforming a long-held goal of organoid developers into a practical reality, which has far-reaching implications for how many medications of the future will be produced.
“Every new drug that the pharmaceutical companies develop needs to be evaluated for drug-induced liver toxicity before it can be used in humans. We hope this tool will change the drug development paradigm and lead to safer drugs for all patients,” Takebe says.
The research team led by Takebe includes scientists in Cincinnati and Japan. Their organoid system builds upon discovering a polygenetic risk score that lays out how to predict which drugs are likely to cause liver injury and which people are most likely to face drug-induced liver injury (DILI). That study appeared in early September, 2020, in Nature Medicine.
The liver organoid system is based on 10 lines of pluripotent liver stem cells that can be grown into functional, 3D versions of human livers. Each organoid possesses the tissues that can transport bile acids like a full-sized liver, which allows a level of analysis that was previously only possible in animal models.
Unfortunately, mice and other animal models often do not precisely mimic what can happen when a human takes a medication. Takebe and colleagues developed this system so that it can be “programmed” via by CRISPR-Cas9 based gene editing to reflect specific disease conditions, including the exact mimicking of a specific patient’s liver.
The result: a living test platform on a plastic chip with 384 wells–each containing a liver organoid–that can provide high-speed results to scientists evaluating variations in drug compounds intended to treat disease. Before having a test like this, liver toxicity complications from new medications often went undetected until the latest stages of the drug testing and approval process.
“Pharmaceutical companies lose significant amounts of time, labor and money developing new drugs that fail at unpredictable times due to liver injury occurring during clinical trials,” Takebe says.
Next Steps
“We will work closely with industry partners and regulatory agencies to accelerate further development and validation of this emerging technology to enable its incorporation into the drug discovery and development process,” says Magdalena Kasendra, Director of Research and Development. “This work will be performed at a newly launched CuSTOM Accelerator focused on the bench-to-bedside translation of organoid technology.”
About this study
This work was supported by grants from the National Institutes of Health (UG3 DK119982), the Cincinnati Children’s Research Foundation, New York Stem Cell Foundation, the Ohio Third Frontier Technology Validation and Start-Up Fund, the Japan Science and Technology Agency, AMED, the Cincinnati Center for Autoimmune Liver Disease, the Digestive Disease Research Core Center at Cincinnati Children’s, the Takeda Science Foundation and the Mitsubishi Foundation.
Read more in Scientific Reports about developing standardized, non-animal origin media for organoid production
Connect with Innovation Ventures to learn more about stem cell and organoid medicine technology at Cincinnati Children’s.
Learn more about the CuSTOM Accelerator.
| Original title: | High-Fidelity Drug Induced Liver Injury Screen Using Human PSC-derived Organoids |
| Published in: | Gastroenterology |
| Publish date: | Oct. 8, 2020 |
Research By






