Every Day is Rare Disease Day at Cincinnati Children’s
Post Date: February 28, 2020 | Publish Date:

As the world of medicine marks Feb. 29 as Rare Disease Day 2020, the clinical specialists and research experts at Cincinnati Children’s continue working every day to improve outcomes for children living with rare conditions.
Here, rare diseases aren’t rare at all.
In the United States, a disease or disorder is defined as “rare” when it affects fewer than 200,000 people at a given time. In Europe, the definition of “rare” applies to diseases affecting fewer than one in 2,000 people.
Some rare diseases are well-known, such as cystic fibrosis, Rett syndrome or Tay-Sachs disease. Others carry less familiar names, such as eosinophilic esophagitis (EoE), 22q13 deletion syndrome, and many others.
Overall, scientists have discovered about 7,000 rare diseases, which makes them a major health challenge when considered together. When combined, an estimated 25 to 30 million people in the U.S. are living with a rare disease. That’s about one of every nine people.
Cincinnati Children’s serves as one of the world’s leading centers in the research and treatment of numerous rare diseases. Read on to learn more about collaborations we support to improve outcomes for children with rare diseases.

A Growing Role for Cincinnati Children’s in Rare Disease Research
In October 2019, Cincinnati Children’s was awarded a five-year, $28 million grant from the National Center for Advancing Translational Sciences (NCATS), to serve as the data management and coordinating center for the federal Rare Diseases Clinical Research Network (RDCRN).
Read more about Cincinnati Children’s new role
Learn more about the network’s Rare Disease Day activities

An Engine of Partnership to Tackle Eosinophilic Disorders
One of the 23 disease-specific networks supported by the RDCRN is based here at Cincinnati Children’s, where Marc Rothenberg, MD, PhD, has worked for years as a pioneer in understanding eosinophilic disorders.
The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) was formed in 2014 to connect families and scientists from around the world to accelerate discovery. As researchers have progressed from understanding the genetic and molecular fingerprints of these conditions to developing promising treatments, the CEGIR network has kept families involved from early on.
“We feel included in every aspect of CEGIR, from the very first conference call to the first clinical trial—which was a study on the food elimination diet. When we shared our viewpoint, they changed their direction based on our feedback. They asked for our perspective because they care and believe that we have valuable knowledge,” says Ellyn Kodroff, President of the Campaign Urging Research for Eosinophilic Disease (CURED) Foundation.
Read more from family advocates about CEGIR

Family Fundraising Drives Scientific Advance in Mitochondrial Disorder
Five-year-old Hadley Jo Brindley has a condition so uncommon it has more of a label than a name: hereditary motor and sensory neuropathy, type VIB—also called HMSN6B for short. In essence, a malfunction in Hadley’s mitochondria—the energy-processing component of all our cells—is gradually affecting her eyesight, limiting her ability to walk, and interfering with her brain development.
Her family’s journey led to Cincinnati Children’s, where research led by Taosheng Huang, MD, PhD, and published Jan. 16, 2020, in the journal Human Molecular Genetics, reveals that a treatment might be possible.
Learn more about how this family partnered with the medical center to raise more than $80,000 to fund ongoing research.

New grants fund work to defeat two rare forms of childhood cancer
Maryam Fouladi, MD, MSc, Medical Director of the Brain Tumor Center here, was awarded a three-year $750,000 grant to fund a Phase 1 study of PTC596 for the treatment of diffuse intrinsic pontine glioma & high-grade gliomas.
Parinda Mehta, MD, Division of Bone Marrow Transplantation and Immune Deficiency, was awarded a four-year $1.7 million grant to fund a Phase 2 study of quercetin chemoprevention for the treatment of squamous cell carcinoma in patients with Fanconi Anemia.
The FDA awarded the grants through its Orphan Products Clinical Trials Grants Program.
Read more

A Knowledge Hub for Smith-Kingsmore Syndrome
When 17 of the estimated 50-60 known families with children diagnosed with Smith-Kingsmore syndrome traveled from multiple countries to gather at Cincinnati Children’s in October 2019, it was a special event.
Not only did far-flung families find new ways to connect, they helped scientists here kickstart a growing collection of data and biosamples that eventually could lead to a clinical trial of a treatment that has shown an initial level of promise.
The 2-day gathering was the brainchild of human genetics expert Carlos Prada, MD, and a team of colleagues at Cincinnati Children’s who have been working closely with families.
“We want to blaze a trail here. We know that SKS is underdiagnosed, but we don’t know if there are another 1,000 people or 100,000 people,” says Mike Groseclose, who worked with his spouse Kristen to launch the Smith-Kingsmore Syndrome website, in 2018 shortly after their son Jack was diagnosed with SKS.
“It was awesome to learn more about the progress made so far. Even though it’s in its infancy, the science is much further along than we understood. It was great for everybody to hear that,” Mike says.
Read more about the SKS story.
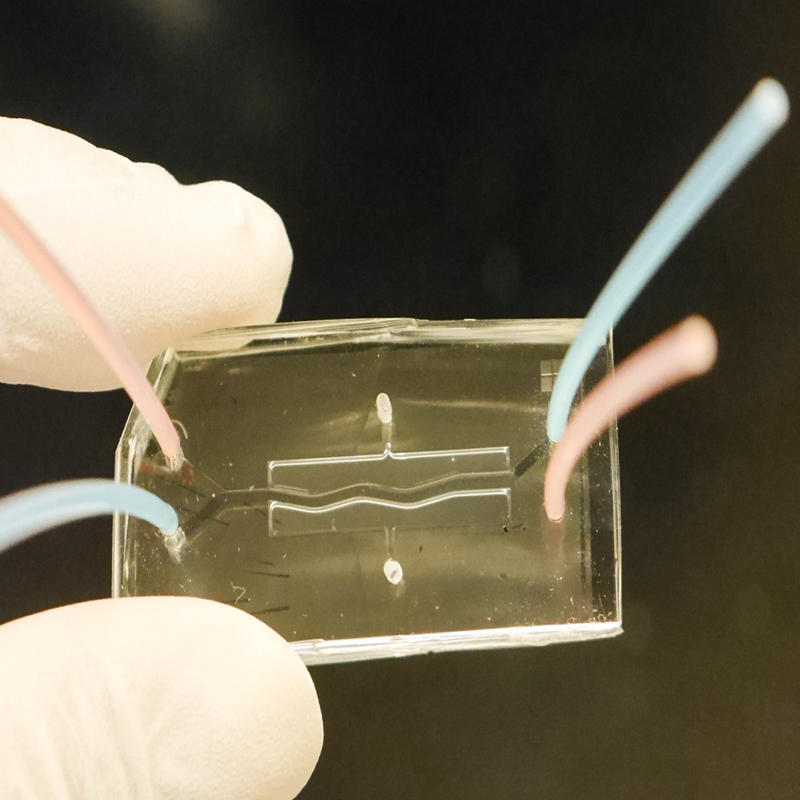
Human Pancreas on a Chip to Help Scientists Study Cystic Fibrosis and Other Diseases
Clinicians and scientists here have been working for years to improve outcomes for children growing up with cystic fibrosis (CF).
Cincinnati Children’s is one of only 10 specially-designated CF Foundation Research Centers in the United States. Experts here have played important roles in many lines of research, including pre-clinical and clinical study of the drugs Kalydeco (approved in 2012) and Trikafta (approved in 2019).
More recently, Anjaparavanda Naren, PhD, Director of our Cystic Fibrosis Research Center, led a team that created a human pancreas on a chip, which allowed them to identify the possible cause of a frequent and deadly complication called CF-Related Diabetes (CFRD).
The small two-chambered device contains tiny human pancreatic organoids that react to potential treatments in a more realistic way than animal models can. Eventually, the chip may also be useful in research for type 1 and 2 diabetes and other conditions.






