Emapalumab Induces Remission in Macrophage Activation Syndrome
Research By: Alexei Grom, MD
Post Date: March 31, 2023 | Publish Date: Mar. 31, 2023
Rheumatology | Top Scientific Achievement

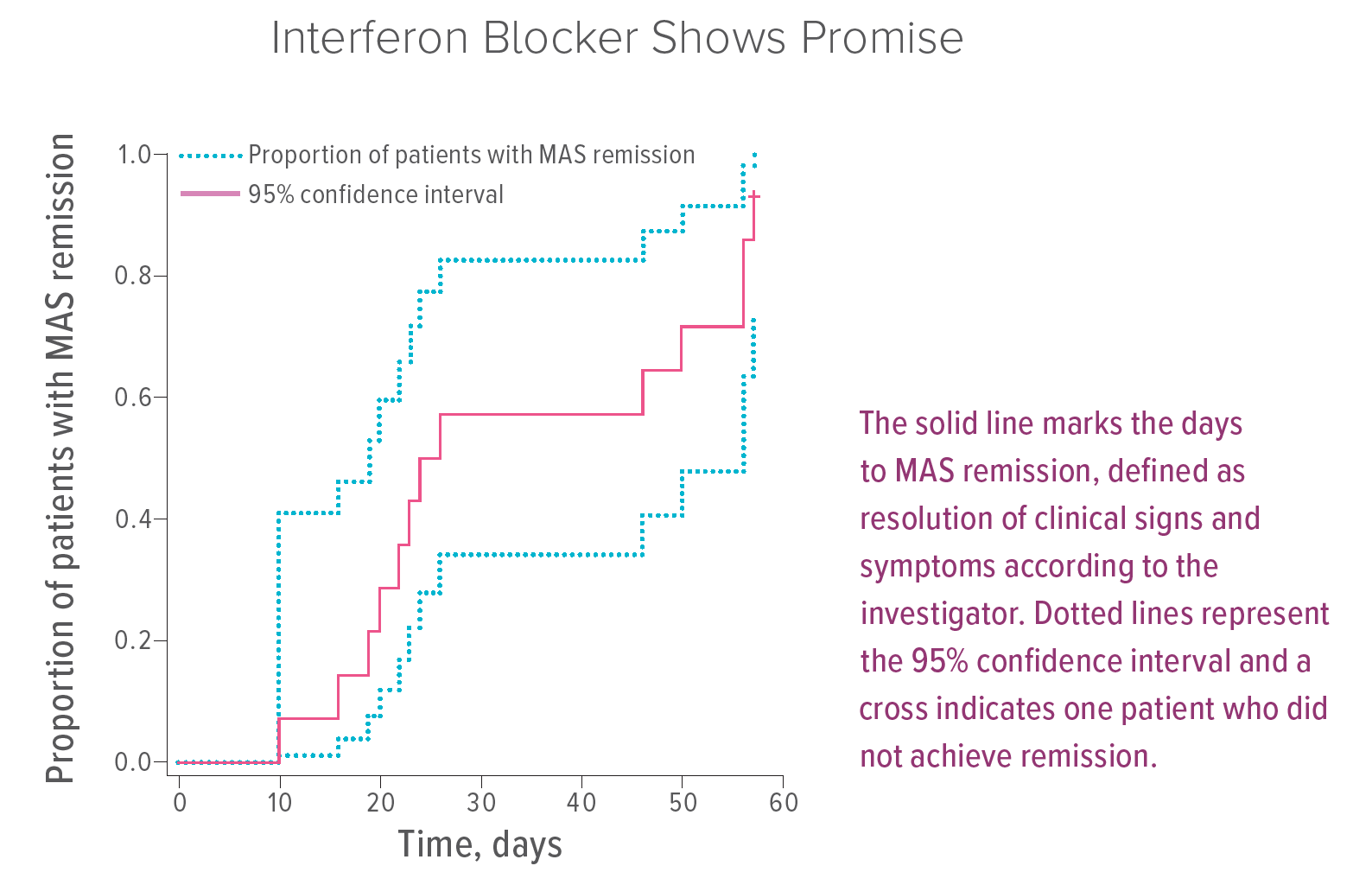
In a clinical trial, neutralization of IFNγ with emapalumab effectively induced remission in 13 of 14 patients with macrophage activation syndrome (MAS) secondary to sJIA/AOSD who failed standard of care including high-dose glucocorticoids with or without anakinra and/or ciclosporin.
Alexei Grom, MD, research director of the Division of Rheumatology at Cincinnati Children’s, was among co-authors from Italy, France, Spain, England, Switzerland and Belgium who conducted a clinical trial to evaluate the treatment benefits of the interferon blocker emapalumab.
Grom also served on the clinical trial’s steering committee. Translational studies that provided the scientific basis to launch the clinical trial were conducted at Cincinnati Children’s in collaboration with Fabrizio De Benedetti, MD, PhD, at the Children’s Hospital in Rome, Italy.
MAS is a life-threatening form of secondary hemophagocytic lymphohistiocytosis (HLH) that can occur as a complication of systemic juvenile idiopathic arthritis (sJIA) and adult-onset Still’s disease (AOSD). Up to 20% of sJIA/AOSD patients develop MAS during their lives. Currently, high-dose steroids are the first-line treatment, but the condition is fatal in about 20% of cases.
“This is the first therapy to be prospectively evaluated for severe MAS that has failed to respond to conventional treatment,” Grom says. “These results, combined with previous trials showing the efficacy of emapalumab in primary HLH, suggest that IFNγ is an important driver of MAS/HLH, independent of the underlying predisposing condition.”
In most cases, patients responded rapidly to the interferon blocker. However, emapalumab does not appear to prevent all flares experienced by sJIA patients, suggesting that continued treatment with anakinra should be considered. Attention also should be paid to the risk of viral infections after receiving the antibody treatment, Grom says.
More 2023 Research Highlights
Chosen by the Division of Rheumatology
Brunner HI, Foeldvari I, Alexeeva E, et al. Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: a randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann Rheum Dis. 2023;82(1):154-160. doi:10.1136/ard-2022-222849
McCracken C, Shantha JG, Yeh S, et al. Relapse and Remission in Children With Chronic Noninfectious Uveitis Treated With Methotrexate. J Rheumatol. 2022;49(11):1289-1291. doi:10.3899/jrheum.220007
Vega-Fernandez P, Oberle EJ, Henrickson M, et al. Musculoskeletal Ultrasound and the Assessment of Disease Activity in Juvenile Idiopathic Arthritis. Arthritis Care Res (Hoboken). 2023;75(8):1815-1820. doi:10.1002/acr.25073
Macaraeg M, Schulert GS. Complications of complications: diagnosis and treatment of recurrent macrophage activation syndrome in a patient with well-controlled systemic juvenile idiopathic arthritis. RMD Open. 2023;9(1):e002611. doi:10.1136/rmdopen-2022-002611
View more discoveries from 50 research divisions and areas
Return to the 2023 Research Annual Report main features
| Original title: | Efficacy and safety of emapalumab in macrophage activation syndrome |
| Published in: | Annals of the Rheumatic Diseases |
| Publish date: | Mar. 31, 2023 |