Brady, Frenck Among Co-Authors of Study on COVID Vax Effectiveness Against Omicron Variant
Post Date: July 21, 2022 | Publish Date:

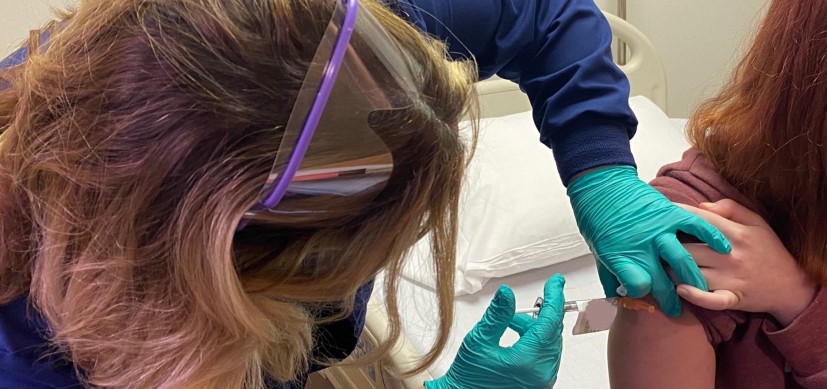
Cincinnati Children’s vaccine experts Rebecca Brady, MD, and Robert Frenck, MD, are among co-authors of a new manuscript reporting that COVID-19 booster doses result in high levels of neutralizing antibodies against the Omicron variant, but the study noted that antibody levels decrease substantially within three months.
The findings, published July 19, 2022, in Cell Reports Medicine, are from an ongoing Mix and Match clinical trial. The study is sponsored by the National Institute of Allergy and Infectious Diseases, which is part of the National Institutes of Health.
Brady, a professor in the Division of Infectious Diseases and director of Adult Clinical Studies at the Gamble Vaccine Research Center, is the site principal investigator for the part of the study conducted at Cincinnati Children’s.
Frenck, a professor in the Division of Infectious Diseases and director of the Gamble Vaccine Research Center at Cincinnati Children’s, is co-investigator.
Preliminary results of the Mix and Match clinical trial, published Jan. 26, 2022, in the New England Journal of Medicine, concluded that COVID-19 booster doses do not have to be made by the same manufacturer as previous doses to be safe and effective at prompting an immune response. Nine different vaccine combinations were studied, using Moderna, Pfizer-BioNTech and Janssen (Johnson & Johnson) vaccines.
The new analysis found that nearly all of the vaccine combinations resulted in high levels of neutralizing antibodies to the Omicron BA.1 sub-lineage. But antibody levels against Omicron were low in the group that received the Johnson & Johnson vaccine as both a primary vaccine and a boost.
In all combinations, the immune responses to Omicron waned, with neutralizing antibody levels decreasing 2.4- to 5.3-fold within three months of a boost. In addition, Omicron sub-lineages BA.2.12.1 and BA.4/BA.5 were 1.5 and 2.5 times less susceptible to neutralization compared to the BA.1 sub-lineage, as well as 7.5 and 12.4 times less susceptible relative to the ancestral D614G strain.
The researchers said the findings demonstrate that novel Omicron sub-lineages are becoming less susceptible to original COVID-19 vaccines, which supports consideration of more targeted booster options.
It has yet to be determined whether the rate of decline of neutralizing antibodies against Omicron can be improved with additional mix or match vaccine boosting or with a longer interval between inoculations, the researchers stated.
Omicron (B.1.1.529) is now the dominant circulating variant amid the COVID-19 pandemic, the researchers noted. A large number of mutations, including about 30 in the spike protein, resulted in increased transmissibility, partial resistance to natural- and vaccine-induced immunity, and increased vaccine breakthrough infections. The BA.4 and BA.5 sublineages are becoming the dominant variants in the pandemic.
The 10-site trial included 458 healthy adults who had already received their primary shots with one of three COVID-19 vaccines and had no reported history of infection before receiving a booster injection with either the Moderna, Pfizer-BioNTech, or Janssen (J&J) vaccine.
A single booster dose was administered to each participant in the trial: 150 received the J&J Ad26.COV2.S vaccine; 154 received Moderna’s mRNA-1273 vaccine; and 154 received Pfizer-BioNTech’s BNT162b2 vaccine. Depending on which primary vaccine regimen a participant had received, the booster vaccine was either different from (mixed, or heterologous) or the same (matched, or homologous) as the original vaccine. Participants were enrolled from May 29 to August 13, 2021.
The new manuscript, titled “Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant,” included input from Brady, Frenck and 35 other co-authors from institutions across the United States.
Cincinnati Children’s Gamble Vaccine Research Center is one of the top sites in the nation for the testing, evaluation, and development of vaccines. Including adults, over 1,500 individuals are participating in one of the 11 COVID vaccine clinical trials at Cincinnati Children’s. That includes Pfizer/BioNTech vaccine as well as vaccines manufactured by Moderna, AstraZeneca, and CyanVac.
More information about the trial is available at ClincalTrials.gov under study identifier NCT04889209.
Read more about the Mix and Match study