Brady, Frenck Among Co-Authors of COVID Vax ‘Mix-and-Match’ Study in NEJM
Post Date: January 26, 2022 | Publish Date: Jan. 26, 2022
Clinical trial sponsored by NIAID finds additional dose is safe and immunogenic even if vaccine products vary
Cincinnati Children’s vaccine experts Rebecca Brady, MD, and Robert Frenck, MD, are among co-authors of a new manuscript reporting that COVID-19 booster doses do not have to be made by the same manufacturer as previous doses to be safe and effective at prompting an immune response.
Preliminary results of the Mix and Match clinical trial were published Jan. 26, 2022, in the New England Journal of Medicine. The data are the first to be published from an ongoing Phase 1/2 trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
Brady, a professor in the Division of Infectious Diseases and director of Adult Clinical Services, is the site principal investigator for the part of the study conducted at Cincinnati Children’s.
Frenck, a professor in the Division of Infectious Diseases and director of the Gamble Vaccine Research Center at Cincinnati Children’s, is co-investigator.
The manuscript, titled “Heterologous SARS-CoV-2 Booster Vaccinations – Preliminary Report,” included input from 36 other co-authors from institutions across the United States.

The 10-site trial included 458 healthy adults who had already received their primary shots with one of three COVID-19 vaccines and had no reported history of infection before receiving a booster injection with either the Moderna, Pfizer BioNTech, or Janssen (J&J) vaccine. Nine different vaccine combinations were studied.
The authors concluded that both heterologous (mixed) and homologous (matched) booster vaccinations were safe and immunogenic in adults who completed a primary Covid-19 vaccine regimen at least 12 weeks earlier.
On the basis of data presented in the report, the U.S. Food and Drug Administration authorized use of these three vaccines for booster doses on a mixed or matched basis.
A single booster dose was administered to each participant in the trial: 150 received the J&J Ad26.COV2.S vaccine; 154 received Moderna’s mRNA-1273 vaccine; and 154 received Pfizer-BioNTech’s BNT162b2 vaccine. Depending on which primary vaccine regimen a participant had received, the booster vaccine was either different from (mixed, or heterologous) or the same (matched, or homologous) as the original vaccine.
All combinations of primary and booster vaccine resulted in increased neutralizing antibody levels (ranging from 4.2- to 76-fold higher levels than those detected prior to boost.) Likewise, all primary-boost combinations increased binding antibody levels 4.6- to 56-fold.
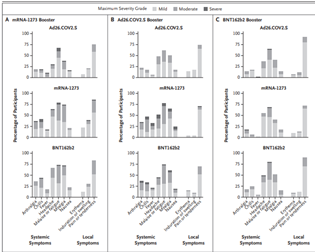
Participants were enrolled from May 29 to August 13, 2021. Immunogenicity was assessed on Day 1 before booster vaccination and at Days 15 and 29 after boost. Reactogenicity was similar to that reported for the primary series.
Most adverse events were mild or moderate. Injection site pain, malaise, headache, and myalgia occurred in more than half of the participants. No serious vaccine-related adverse events were reported.
Do you have questions about COVID-19 vaccines? Visit our information page for answers
The authors noted that the study had limitations. For example, it was not designed to directly compare responses between different booster regimens and did not include an un-boosted control group.
Investigators will continue to follow participants for one year to assess what impact booster vaccination has on longer-term immune responses.
Cincinnati Children’s Gamble Vaccine Research Center is one of the top sites in the nation for the testing, evaluation, and development of vaccines. Including adults, over 1,500 individuals are participating in one of the 11 COVID vaccine clinical trials at Cincinnati Children’s. That includes Pfizer/BioNTech vaccine as well as vaccines manufactured by Moderna, AstraZeneca, and CyanVac.
Read more about COVID-19 research from experts at Cincinnati Children’s
Predictors of Severe Outcomes in Children with COVID-19
Paulsen, Frenck Co-Author NEJM Report Detailing Clinical Trial Data for Child-Sized COVID-19 Vax
Vaccine ‘Highly Effective’ in Preventing Adolescent COVID-19 Hospitalization
7 COVID Findings Among Most Shared Science of March 2021
Nasal Vaccine to Battle COVID-19 Begins Clinical Trial at Cincinnati Children’s
How COVID-19 Reinforces Known Health Disparities
| Original title: | Homologous and Heterologous Covid-19 Booster Vaccinations |
| Published in: | The New England Journal of Medicine |
| Publish date: | Jan. 26, 2022 |