Adult Med Also Reduces Monthly Migraine Days for Many Children
Research By: Andrew Hershey, MD, PhD
Post Date: January 14, 2026 | Publish Date: Jan. 15, 2026
Cincinnati Children’s expert served as lead investigator for SPACE clinical trial. Results published in NEJM.
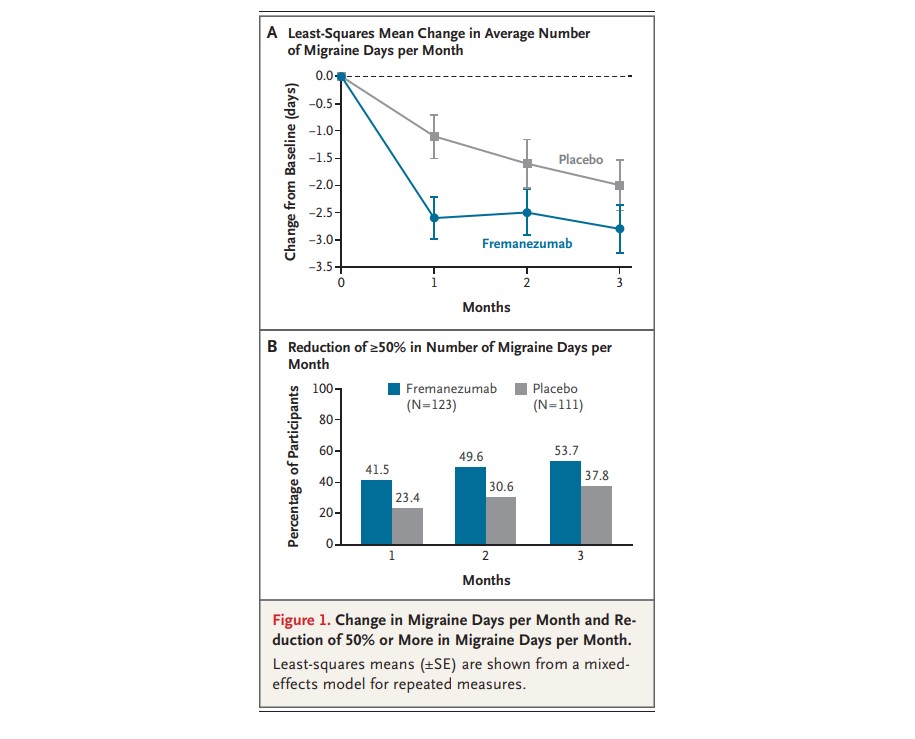
Monthly injections of fremanezumab (brand name AJOVY) helped 47% of children taking the drug cut their number of migraine days in half, according to clinical trial results published Jan. 15, 2026, in The New England Journal of Medicine.
The SPACE clinical trial enrolled 237 children ages 6-17 to evaluate the medication made by Teva Pharmaceuticals. The data contributed to the U.S. Food and Drug Administration (FDA) approving AJOVY for use in August 2025 in this pediatric age group as long as they weigh 45 kilograms (99 pounds) or more. The FDA initially approved the drug for adult use in 2018.
The NEJM study reports that the medication reduced monthly migraine days by 2.5 days compared to 1.4 days with placebo. Meanwhile, the percentage of participants achieving at least a 50% reduction in migraine days was 47.2% among the medication group compared to 27% among the placebo group.
The FDA approval makes AJOVY the first and only calcitonin gene-related peptide (CGRP) antagonist indicated for preventive treatment of episodic migraine in pediatric patients.
“Helping to prevent migraine attacks in children and adolescents is critical to supporting their healthy development, education and overall social well-being,” says Andrew Hershey, MD, PhD, director, Division of Neurology at Cincinnati Children’s. “The SPACE trial demonstrates that a CGRP-targeted preventive therapy like AJOVY can significantly reduce episodic migraine frequency in young patients, giving physicians critical evidence to guide care for this underserved population.”
In 2017, Hershey and colleague Scott Powers, PhD, published a major finding in NEJM that two leading migraine medications at the time were no more effective than placebo at controlling migraine. Since then, the researchers have published positive results from cognitive behavioral therapy techniques and Hershey supported another study showing in-school benefits from an arm device.
AJOVY represents a newer class of medications that has begun reaching the market.
“While the older medications we evaluated showed limited benefit in children, this new class of CGRP antagonists has demonstrated more impressive result,” Hershey says. “Whether these new medications outperform cognitive behavioral therapy has not been evaluated. But some patients with migraine may have difficulty accessing or staying with CBT. It is helpful to have FDA-approved options for such patients.”
See the full announcement from Teva Pharmaceuticals
Learn more about migraine management from the Headache Center at Cincinnati Children’s
Don’t Miss a Post:
- Subscribe to the Research Horizons Newsletter
- Follow Cincinnati Children’s Research Foundation on Bluesky, X and LinkedIn
| Original title: | Fremanezumab in Children and Adolescents with Episodic Migraine |
| Published in: | The New England Journal of Medicine |
| Publish date: | Jan. 15, 2026 |
Research By

My research seeks to understand the clinical and biological characteristics of headaches in order to improve the outcomes of not only the patients and families we see, but of patients everywhere.