Two-Hit Model of Muscular Dystrophy May Redirect Hunt for Cures
Research By: Jeffery Molkentin, PhD
Post Date: February 8, 2023 | Publish Date: May 26, 2022

Scientists discovered in 1986 that common muscular dystrophies (MDs) could be traced to specific mutations of a single gene: dystrophin (DMD). Dramatic breakthroughs in curative treatment seemed just around the corner.
Slow scroll forward three decades.
It took until 2016 for the first in a class of “exon-skipping” treatments of the DMD gene to win FDA approval (eteplirsen). Since then, three more similar-acting drugs have been approved (golodirsen, viltolarsen, and casimersen). The drugs are predicted to help slow disease progression for up to 30% of people with muscular dystrophies. They are not considered cures, according to CureDuchenne.org.
Now, a study led by experts at Cincinnati Children’s sheds light on why the hunt for cures has been so sluggish. The findings were so surprising to peer reviewers that it took three years for the paper to move from preprint to final publication in Nature Communications.
“The MDs as a group are most typically associated with genetic mutations in genes that underlie some aspect of plasma membrane support, repair, or the activity of select channels and signaling components within the membrane,” writes lead author Justin Boyer, PhD. “Our results suggest that these underlying genetic defects that comprise the MDs only represent the first ‘hit’ in a ‘two-hit’ model, with the second hit being that of additional destabilization associated with induction of the myogenic fetal gene program due to new satellite cell (muscle stem cells) activity and myoblast fusion.”
In essence, the research team found yet another double-edged sword amid the workings of the human genome—this one involving the mechanisms of muscle tissue healing. The discovery came about in an unexpected fashion.
Initially, the team was examining the opposite hypothesis, mainly that greater induction of muscle stem cells would protect from MD by causing more regeneration. However, inducing greater stem cell activity and the attempts at repair further destabilized the myofiber plasma membrane, which synergized with the loss of dystrophin.

WHEN GOOD SATELLITE CELLS CAUSE HARM
The MDs are genetic disorders that share progressive muscle deterioration and weakness as distinguishing symptoms.
In general, the most common form of MD, Duchenne MD, is diagnosed between ages 3 and 5 and the deterioration worsens over time. This form of MD occurs in about one of every 3,600 male births worldwide. It affects males because the dystrophin gene is on the X chromosome.
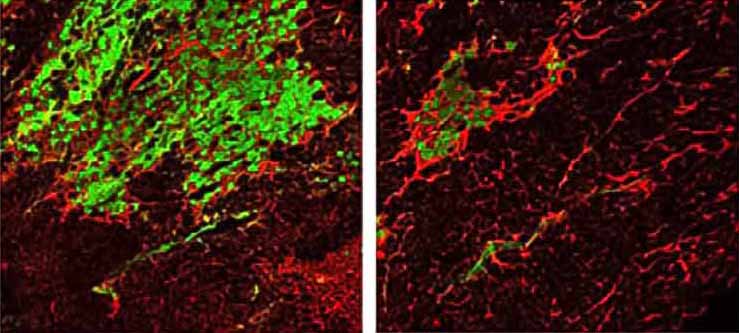
For most people, muscle injury healing is spurred along by muscle satellite cells that function to regenerate the tissue. But for people with MD, this study shows that satellite cells actually contribute to muscle wasting.
Boyer and colleagues determined that this natural repair program, whereby newly activated satellite cells fuse into existing damaged myofibers, requires weakening of the plasma membrane, which when coupled with the loss of dystrophin, causes MD. That’s the two-hit hypothesis.
IN MICE, UNWANTED REPAIR PROCESS CAN BE TURNED OFF
“By depleting satellite cells and their induction of the MyoD-dependent fetal gene program in myofibers using either of two separate genetic approaches in two different mouse models of MD, we demonstrate an overwhelming effect on myofiber plasma membrane stability,” the co-authors state.
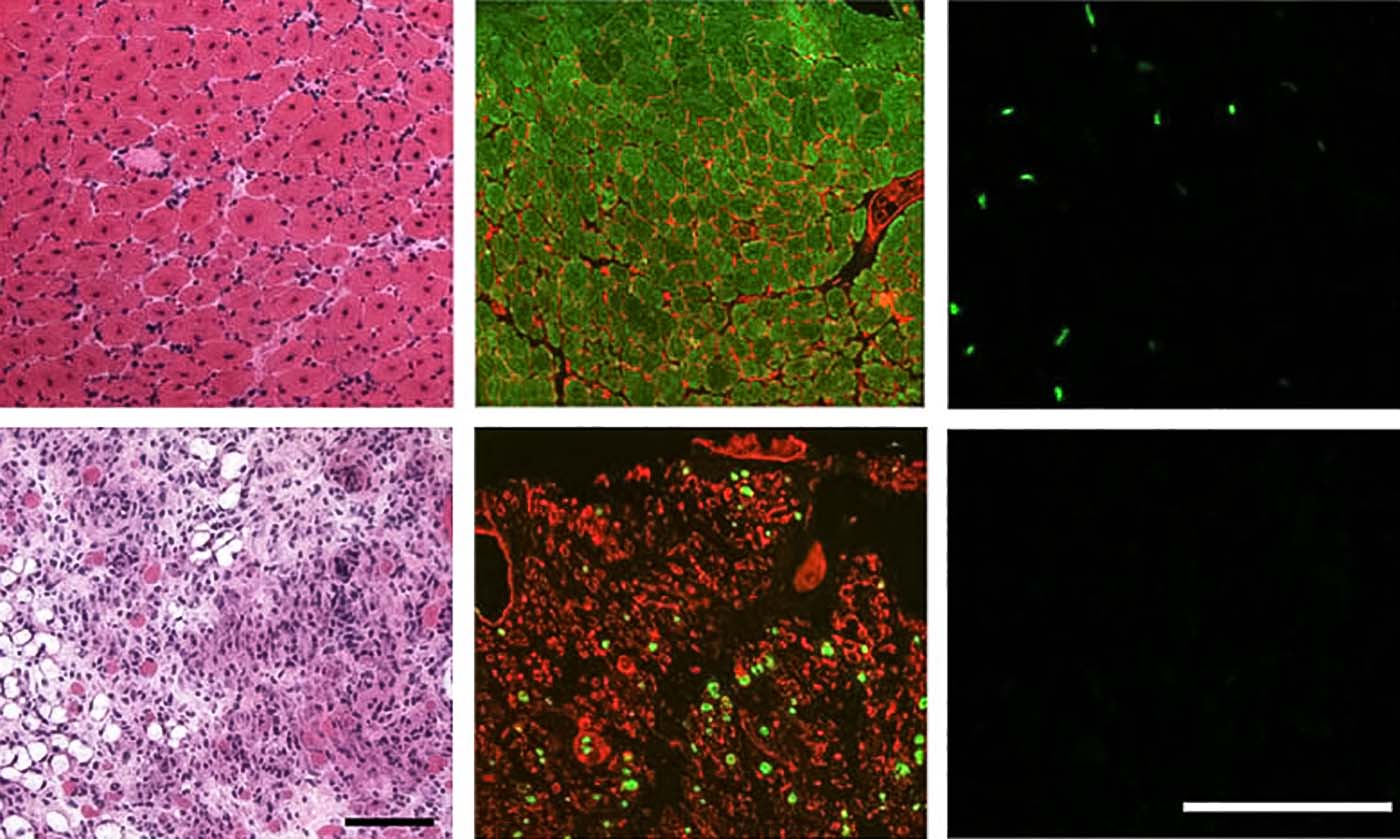
The paper details how the team depleted satellite cell populations in mouse muscle tissue, and how that produced the initially unexpected result of slowing muscle wasting in mice with MD mutations.
“However, a therapeutic approach aimed at depleting satellite cells in MD patients would be ill-advised at this point in time,” says senior author Jeffery Molkentin, PhD, director, Division of Molecular Cardiovascular Biology at Cincinnati Children’s. “But this does not mean that a valuable future treatment is beyond imagination. First, more research is needed to define the most critical molecular effectors of these disease processes, such as new ways of preventing myofiber plasma membrane instability.”
Molkentin presented data from the study in July 2021 at the New Directions in Biology and Disease of Skeletal Muscle Conference held in Charleston, SC. Since the findings were published in May 2022, the paper has been accessed online more than 4,700 times.
Read the 2022 Research Annual Report

ABOUT THE STUDY
In addition to Boyer and Molkentin, co-authors from Cincinnati Children’s and the University of Cincinnati include graduate student Jiuzhou Huo, medical student Sarah Han, MD/PhD student Julian Havens, Vikram Prasad, PhD, Brian Lin, PhD, Taejeong Song, PhD, and Sakthivel Sadayappan, PhD, MBA. The study also included collaborators from Johns Hopkins, the University of Maryland, and Myologica LLC. Funding sources included grants from the National Institutes of Health and a Developmental Award from the Muscular Dystrophy Association.
| Original title: | Depletion of skeletal muscle satellite cells attenuates pathology in muscular dystrophy |
| Published in: | Nature Communications |
| Publish date: | May 26, 2022 |
Research By

Our laboratory investigates a range of focus areas, all of which center on understanding the molecular mechanisms of heart and skeletal muscle disease.







