Small Molecule Exploits Achilles’ Heel of AML, Kills Cancer Cells
Research By: Daniel Starczynowski, PhD
Post Date: March 16, 2022 | Publish Date: March 9, 2022
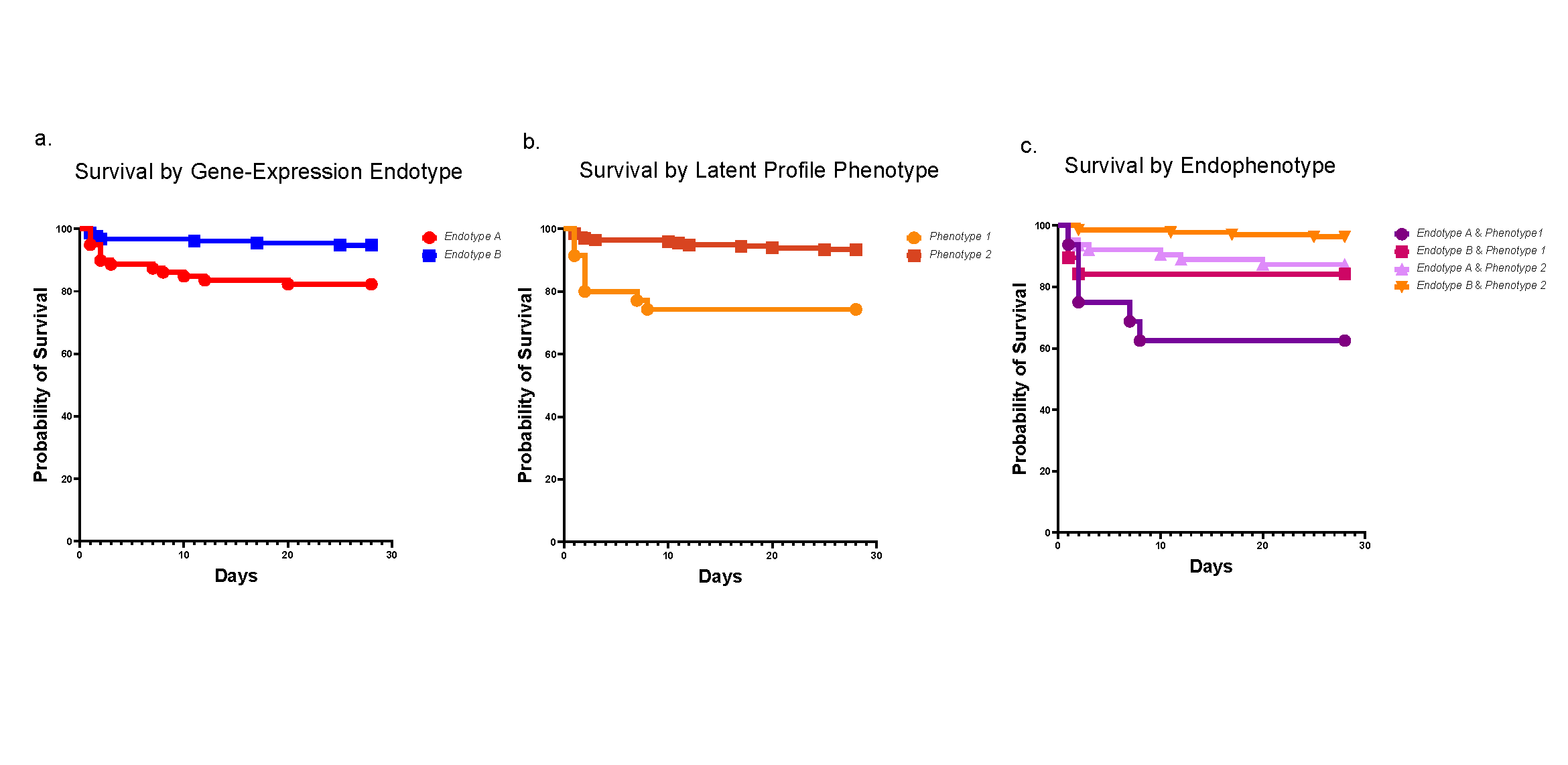
Research led by experts at Cincinnati Children’s has revealed a protein—UBE2N—that appears crucial to multiple pathways that contribute to acute myeloid leukemia (AML) cell survival.
The team also has found a class of small molecules that block the protein’s function, which in turn kills AML cells in lab dishes and in mouse models—without killing healthy blood cells.
This potential breakthrough toward developing a novel therapy for AML was published online March 9, 2022, in Science Translational Medicine. The work was led by co-first authors Laura Barreyro, PhD, and Avery Sampson, BSc, and senior author Daniel Starczynowski, PhD, of the Cancer and Blood Diseases Institute at Cincinnati Children’s. Co-authors include 31 other scientists from Cincinnati Children’s, the University of Cincinnati and five other institutions.
‘Durable’ AML treatments hard to find
“This is novel chemistry that has never been used in medicine for any other reason that we’re aware of,” Starczynowski says. “The team not only identified a new therapeutic target in AML and characterized its mechanism, but they also found a new small molecule that could eventually lead to a new drug.”
Finding efficacious and durable treatments are urgently needed for patients with AML because current treatments for these patients typically have only short-term benefits or none at all.
This discovery builds upon years of work conducted by Starczynowski and colleagues to explore how cancer growth is affected by altered ubiquitination, particularly of proteins implicated in innate immune pathways in leukemic cells, such as from myelodysplastic syndromes (MDS) and AML.
In this case, the team found that several redundant innate immune pathways that AML uses to survive all depend on the function of the protein UBE2N. Cutting off this critical convergence point of escape routes could make it much harder for the cancer cells to expand and survive treatment.
“Not only does the new compound appear to be worth exploring to treat AML, similar dependencies on UBE2N are observed in a number of other conditions,” Starczynowski says. These include solid tumor cancers such as breast, ovarian and colon cancers, as well as some chronic inflammatory disorders.
Next steps
Cincinnati Children’s has filed patent applications for the compound and the Innovation Ventures team here is working to find partners for further development. Whether the next step involves a licensing arrangement, launching a start-up company, or another approach remains undecided.
“We still have a lot more work to do,” Starczynowski says. “This a compound, not a drug. It still needs to be refined and optimized before we can get it to patients.”
Some of Starczynowski’s previous MDS and AML research already has led to forming a start-up company in 2019 called Kurome Therapeutics. That research involved signaling pathways regulated by the genes IRAK1 and IRAK4.
“The new findings about UBE2N are not directly related to but rather complement Kurome’s work,” Starczynowski says.
About the study
Co-authors from Cincinnati Children’s and UC included: Chiharu Ishikawa, BS, Kathleen Hueneman, BSc, Kwangmin Choi, PhD, Mario Pujato, PhD, Somchai Chutipongtanate, MD, PhD, Michael Wyder, MS, Wendy Haffey, PhD, Eric O’Brien, MBA, Mark Wunderlich, BS, Vighnesh Ramesh, BS, Ellen Kolb, BS, Lyndsey Bolanos, BSc, Susanne Christie, BS, Molly Smith, PhD, Madeline Niederkorn, PhD, Tomoya Muto, MD, PhD, Matthew Weirauch, PhD, Zartash Gul, MD, Stephen Medlin, DO, Rhett Kovall, PhD, Kenneth Greis, PhD, and John Perentesis, MD.
Collaborating institutions included Mahidol University in Thailand, Weill Cornell Medical College in New York, the University of Virginia, Providence St. John’s Health in California, and the Albert Einstein College of Medicine in New York.
Funding sources for this study include the Cincinnati Children’s Hospital Research Foundation, the Blood Cancer Discoveries Grant program (sponsored by the Leukemia & Lymphoma Society, the Mark Foundation for Cancer Research. and the Paul G. Allen Frontiers Group), the American Society of Hematology, the Robert Wood Johnson Foundation, the National Cancer Institute and several National Institutes of Health research and training grants (R35HL135787, R01DK102759, R01DK113639, F31HL132420).
| Original title: | Blocking UBE2N abrogates oncogenic immune signaling in acute myeloid leukemia |
| Published in: | Science Translational Medicine |
| Publish date: | March 9, 2022 |
Research By