RSV Vax Trial Seeks to Protect Newborns by Protecting Moms
Post Date: February 3, 2022 | Publish Date:

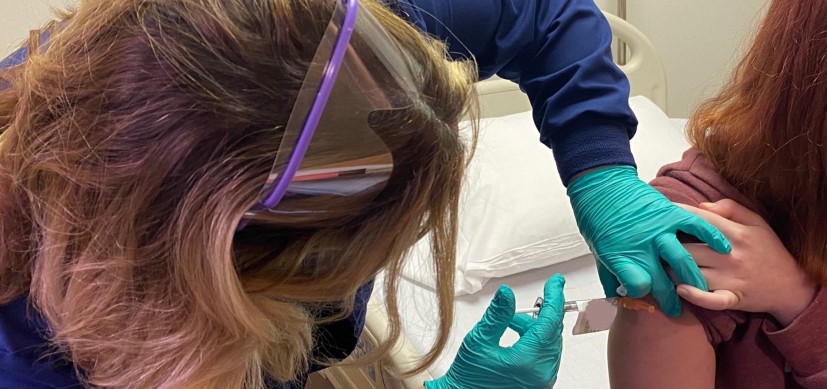
Elizabeth Schlaudecker, MD, MPH, Medical Director of the Division of Infectious Diseases at Cincinnati Children’s, is principal site investigator for a new clinical trial in which pregnant women are vaccinated against respiratory syncytial virus (RSV) in hopes of passing antibodies on to their babies.
RSV is a common illness among children less than 12 months old. Most children in that age range have at least one if not two RSV infections, Schlaudecker said.
That’s a concern because RSV is one of the leading causes of emergency department visits and hospitalizations among babies in the United States, and RSV can even lead to death in infants and children, she noted.
“It is one of our most feared illnesses,” Schlaudecker said. “We see children hospitalized in the intensive care unit. It’s especially (frightening) when babies under 12 months of age are on ventilators or oxygen. That doesn’t make the virus go away; it just supports the baby as they fight off the virus. We don’t have any other treatments for this virus.”
Almost every child gets RSV in the first year or two of life, and it also can affect older kids and adults, Schlaudecker said. A child can easily acquire it from other kids in the neighborhood or at daycare or church.

Respiratory syncytial virus usually surges in winter months, but it began to circulate widely this past July – likely due to some reduction in public health measures to prevent COVID-19. High numbers have been reported for an extended period of time – both in Cincinnati and around the world.
RSV can cause inflammation of the small airways in a baby’s lungs, called bronchioles, leading to bronchiolitis or pneumonia. The lungs become full of mucus and infection-fighting cells to combat the virus, but that sometimes makes it very difficult for a baby to breathe or eat.
Because babies don’t develop their own antibodies until they are 4 to 6 months old, “we feel somewhat powerless as doctors,” Schlaudecker said.
A Gift From Vaccinated Moms: Early Immunity for Newborns
But Phase 3 of Pfizer’s Matisse RSV vaccine trial offers hope for the future. The babies of vaccinated women might have immunity from the moment they are born.
The Pfizer vaccine employs a prefusion protein, which helps activate antibodies in the immune system but doesn’t have any long-term effects. It doesn’t use the mRNA technology that powers Pfizer’s COVID vaccine, and the RSV vaccine also doesn’t include any kind of live virus.
The study, which is being conducted at sites across the United States and around the world, seeks to enroll a total of 10,000 women. The trial involves women under age 49 who expect to deliver babies by mid-March.
Cincinnati Children’s hopes to enroll about 40 women in the local trial, and the enrollment period runs through February. Each participant will receive either the vaccine or a placebo.
Phase 2a of the trial, which involved non-pregnant women, demonstrated that the vaccine was safe and prompted a good immune response. Preliminary data from Phase 2b, which was recently completed, indicated that the vaccine was both effective and safe in pregnant women, Schlaudecker said.
“Phase 3 is exciting from a pediatric standpoint because we will vaccinate pregnant women and then follow their children for a year to make sure the vaccine protects both of them against RSV,” said Schlaudecker, who noted that newborns are too young to be vaccinated.
“Like the flu vaccine and the COVID vaccine more recently, this RSV vaccine will pass from mothers to their babies – both through umbilical cord blood and then hopefully through breastfeeding,” Schlaudecker said.
Proud to play a role in slowing RSV
One of the participants in the trial, Beth, is 38-weeks pregnant with a due date of Feb. 10. She enrolled in the study in December and received either the vaccine or a placebo as a participant.
“That’s something that I was really excited to do,” said Beth, 32, a Cincinnati pharmacist who asked that her last name not be disclosed for privacy reasons. “I could be part of something that would allow us to help pregnant people protect their infants when they’re in a very vulnerable stage.”
Another reason Beth wanted to enroll is because she has a 2-year-old daughter, who is at high risk for picking up the RSV virus at daycare and bringing it home.
“I’m due in February, which is right in the middle of RSV season,” Beth said. “So, if I had the opportunity to be able to potentially get a vaccine that might protect against severe illness for the child that I’m currently pregnant with, that was something that I was very excited to do.
“I know RSV can be potentially life threatening for young children,” Beth added. “I talked to my OB and said, ‘This is something I’m thinking about doing. … Do you think it’s a good idea?’ And they said, ‘Absolutely. Cincinnati Children’s has amazing protocols in place to keep people safe during vaccine studies.’ And they had absolutely no concern about me joining the study.
“My husband’s also a pharmacist, and he also had really no hesitation in me signing up for the study,” Beth added.
More Enrollees Welcome
Enrollment in the Pfizer Matisse RSV vaccine clinical trial at Cincinnati Children’s runs through February, but delivery date must be by about mid-March. Participants are compensated up to $400 for their time and travel. Those who are pregnant can sign up for the study by emailing the study coordinator at IDStudies@cchmc.org or calling (513) 827-8363.
Read More
Info about the MATISSE clinical trial
RSV vaccine during pregnancy could prevent life-threatening respiratory virus in infants (Enquirer)
Local researchers working on RSV vaccine study to protect mother, baby (WKRC)