‘R2’ Herpes Vaccine Shows Promise
Research By: David I. Bernstein, MD, MA
Post Date: November 9, 2020 | Publish Date: Nov. 6, 2020
A modified live virus vaccine against cold-sore producing herpes simplex virus type 1 (HSV-1) generated a powerful antibody response in animal models and demonstrated cross-protection against HSV-2, which leads to genital herpes.
Findings were published Nov. 6, 2020, in the journal npj Vaccines. David I. Bernstein, MD, MA, at Cincinnati Children’s was corresponding author for the study, which included collaborators from Northwestern University and the University of Nebraska-Lincoln.
Despite decades of work, no vaccine has yet succeeded against HSV-1 or HSV-2. Experts estimate that about 400 million people have HSV-2, which can flare up during times of stress throughout an infected person’s life. HSV infections can be especially serious among immunocompromised people and may contribute to Alzheimer’s disease or other forms of dementia.
There are several types of herpes vaccine under evaluation. This one is dubbed “R2” because it modifies a protein in region 2 of the virus’ capsid, a region crucial to helping the virus invade nerve cells.
“The R2 design offers another solution to the challenge of producing a virus that replicates in the periphery inducing a strong immune response without seeding the nervous system and establishing a latent infection,” the study authors state.
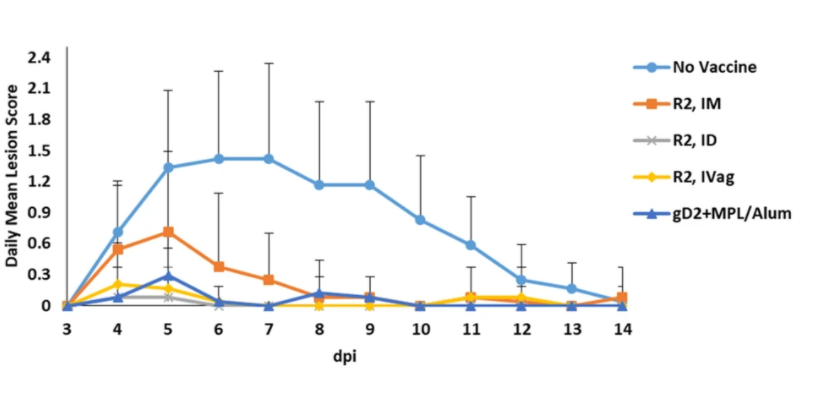
The researchers found that recurrent virus shedding was reduced by 33% to 64% in each of the R2 vaccinated groups whereas the gD2+MPL/Alum control group showed no benefit.
“This is of particular importance because recurrent virus shedding is the main means of HSV transmission,” the co-authors say.
| Original title: | The R2 non-neuroinvasive HSV-1 vaccine affords protection from genital HSV-2 infections in a guinea pig model |
| Published in: | npj Vaccines |
| Publish date: | Nov. 6, 2020 |
Research By