Novel Approach Generates Most Complex Stomach, Esophagus Organoids to Date
Research By: James Wells, PhD | Michael Helmrath, MD | Aaron Zorn, PhD
Post Date: February 8, 2023 | Publish Date: Jan. 6, 2022

In a significant step forward in regenerative medicine, scientists at Cincinnati Children’s report success at developing a stomach organoid so sophisticated that it has distinct glands and nerve cells that can control smooth muscle contractions.
The achievement demonstrates that separate layers and portions of complex organs can be grown from separate lines of human pluripotent stem cells (PSCs) and be combined for continued development. Importantly, the approach used to produce these multi-layered stomach organoids also can be used to make more-complex versions of other lab-grown organs.
“This advance in tissue engineering is important because we can now assemble complex organ tissues from separately derived components, similar to an assembly line approach,” says corresponding author James Wells, PhD.
Co-authors from Cincinnati Children’s include lead author Alexandra Eicher, PhD, Daniel Kechele, PhD, Nambirajan Sundaram, PhD, H. Matthew Berns, DO, Holly Poling, BS, Lauren Haines, BS, J. Guillermo Sanchez, BS, Mansa Krishnamurthy, MD, MSc, Lu Han, PhD, Michael Helmrath, MD, and Aaron Zorn, PhD. Keishi Kishimoto, PhD, from the RIKEN Center for Biosystems Dynamics Research in Japan also was a co-author.
LAYER-BY-LAYER ASSEMBLY
Most organoids made so far can form 3D structures involving multiple cell types. In a lab dish, these tiny organs perform real functions that provide new opportunities to study diseases and develop cures. But they typically lack one or more cell types needed to produce a full-sized functional organ, such as nerve fibers, internal blood vessels, immune cells, and other critical ducts and glands. This new stomach organoid does not yet have every cell type it needs, but it represents a leap forward.
“We started with cells from the three primary germ layers—enteric neuroglial, mesenchymal, and epithelial precursors—all separately derived from PSCs,” Eicher says. “From these we
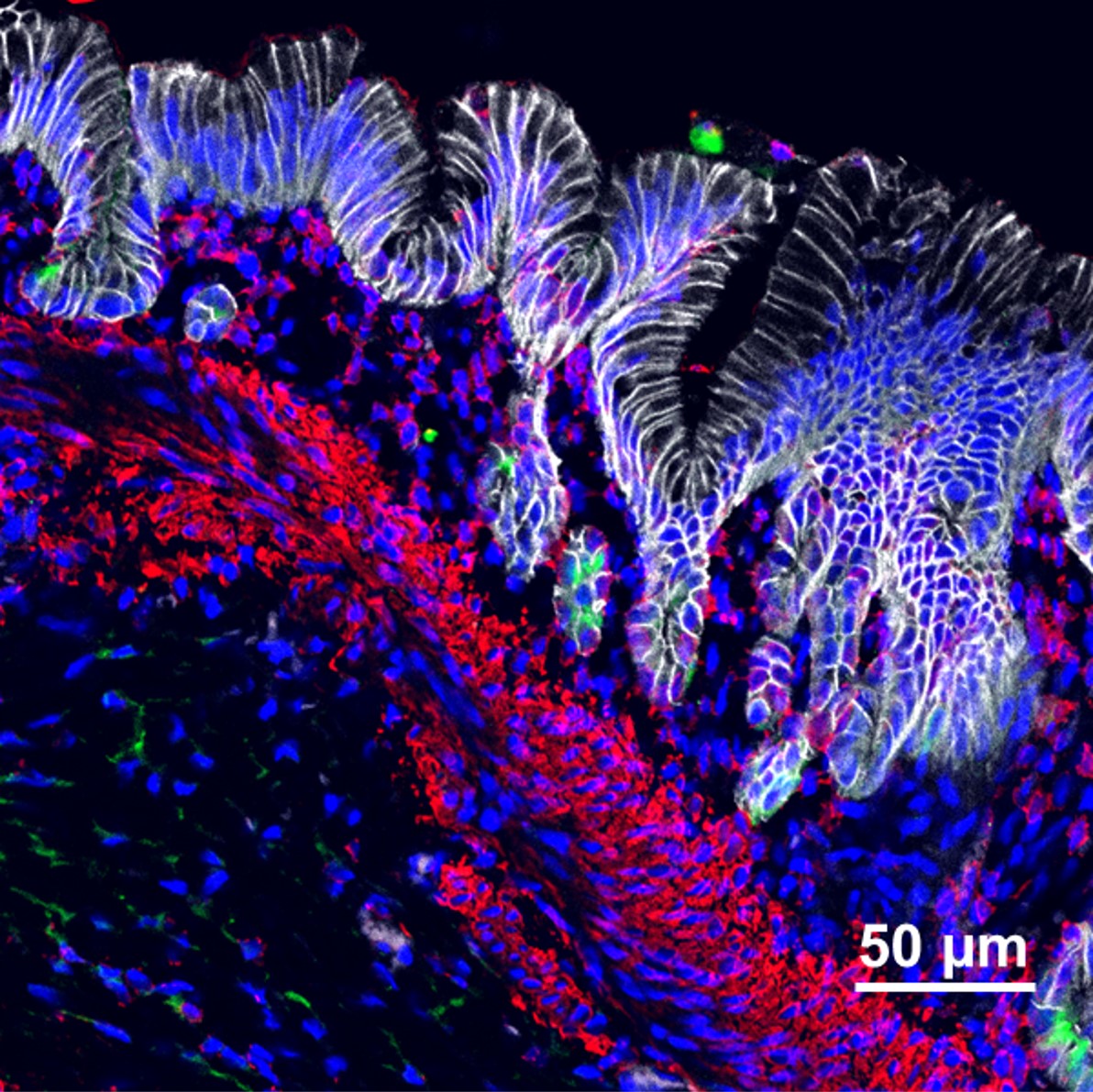
generated stomach tissue that contained acid-producing glands, surrounded by layers of smooth muscle containing functional enteric neurons that controlled contractions of the engineered antral stomach tissue.”
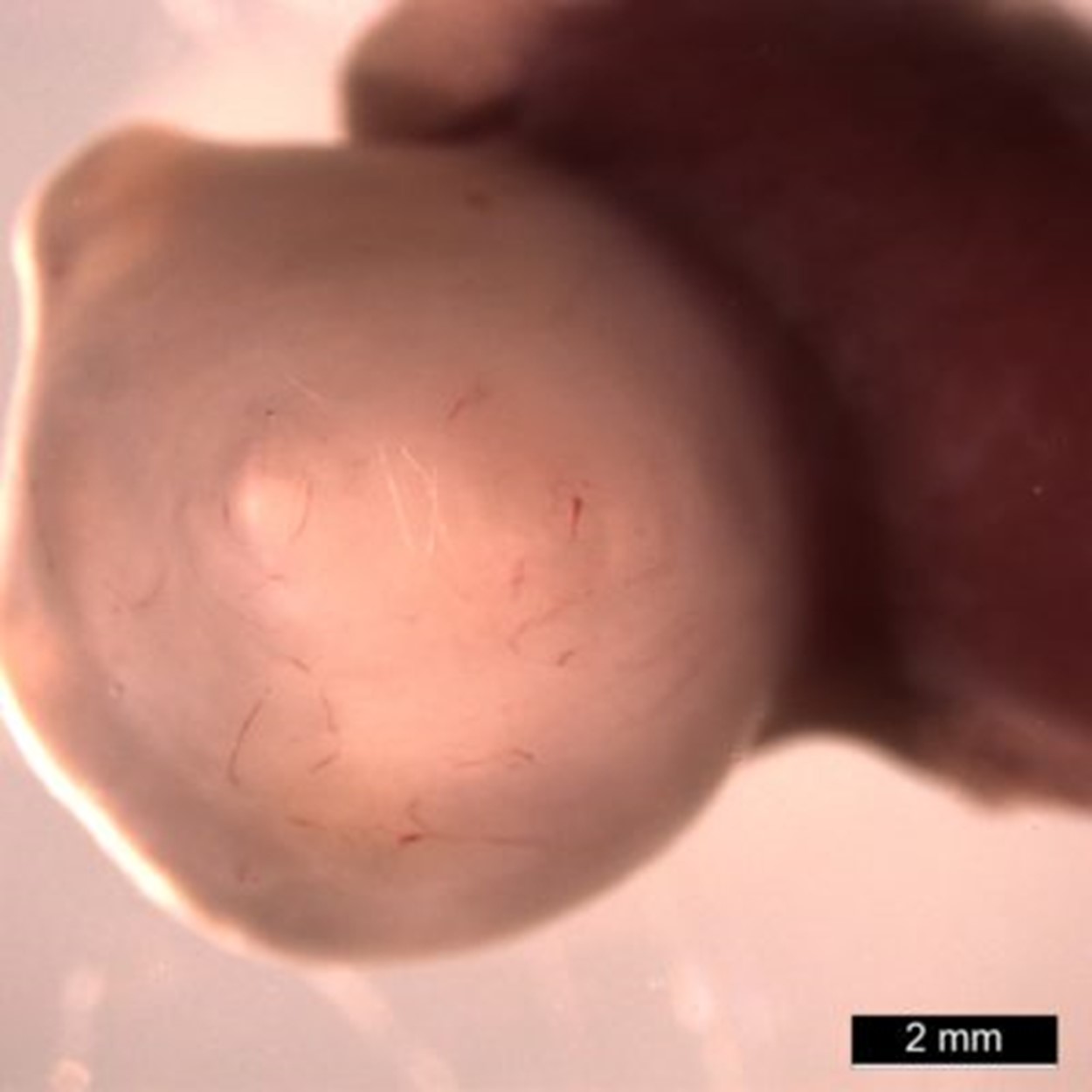
Importantly, the development of these mini human stomachs was not limited to a thin layer of medium in a lab dish. Once the organoids reached a critical stage (at about 30 days) the team performed microsurgery to transplant the organoids into a mouse, which provided the blood flow and biological space to allow much more growth.
Instead of spheres of cells that look like dots in a dish, these organoids grew a thousand-fold in volume inside the mice to form tissues plainly visible to the naked eye. When viewed under a confocal microscope, with different cell types stained to glow in different colors, these organoids radiate a rainbow of complexity.
In fact, the lab-grown tissue closely resembles naturally grown human tissue at similar stages of development. This includes developing a Brunner’s gland, which secretes an alkaline mucus that protects the duodenum from the acidity of stomach contents. The team also discovered that all these cell types and components are needed to generate true stomach tissue because each component helps guide the proper formation of the other components. For example, the authors found that if they did not add the nerves during the assembly process, the stomach glands and muscle tissue did not form properly. Beyond the stomach, the research team also demonstrated a similar approach for developing more sophisticated esophageal organoids.
IMMEDIATE AND LONG-TERM POTENTIAL
At a minimum, these more-complex organoids will serve as useful tools for studying genetic variations and other cell signaling dysfunctions that contribute to gastric diseases. They also will serve as improved platforms for evaluating potential treatments. But there may be even wider-scale impact from these findings.
to other organs, it is possible that engineered tissue might be a source of material for reconstructing elements of the upper GI tract that are damaged by congenital disorders or acute injuries,” Wells says. While much works remains to develop organoid tissue that would be suitable for transplantation, much progress also has been made.
LEADER IN REGENERATIVE MEDICINE
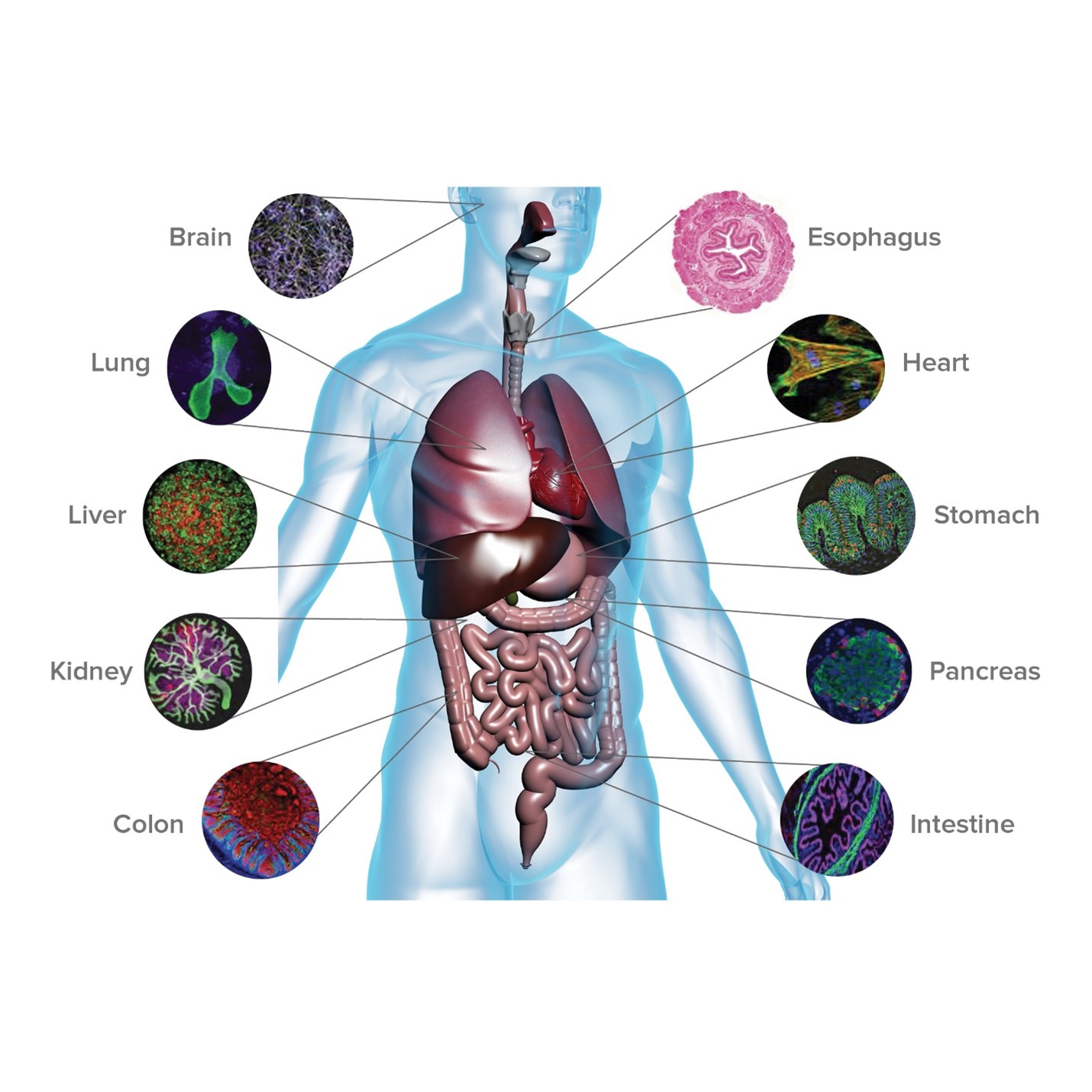
Cincinnati Children’s has played a leading role in organoid research since 2010 when Wells and colleagues published findings in Nature reporting their first success at developing functional intestinal tissue. In 2019, the medical center launched its Center for Stem Cell and Organoid Medicine (CuSTOM) to further accelerate the work. Over the years, the growing team has:
- Added nerves to intestinal organoid tissue
- Demonstrated how to mass-produce liver “buds”
- Produced liver organoids for specific disease states
- Grown both major portions of the stomach
- Developed functional esophagus tissue
- Grown a three-organoid system (liver, pancreas, bile ducts)
INVESTING IN CURES
The Helmrath lab at Cincinnati Children’s is working with 35 other labs in 15 divisions at Cincinnati Children’s to expand organoid development for use in human transplantation.
This line of research is funded in large part through a $10 million grant from Cincinnati Children’s awarded as part of the medical center’s Pursuing Our Potential Together initiative. Support also has come from the Farmer Family Foundation, which helped launch the CuSTOM Accelerator Lab.
More grants, gifts, and investments are needed because growing organoids for clinical purposes requires that the entire process meets Good Manufacturing Practice (GMP) regulations established by the U.S. Food and Drug Administration to assure consistency and safety. That means, for example, the research team needs to find and validate replacements for certain materials used to grow organoids because those materials do not fully meet GMP standards.
“Thanks to all the support we have received so far we have taken several steps needed to scale up production of therapeutic quality organoid tissues. We still have work to do,” Wells says. “Our goal is to achieve transplantation into patients by the end of the decade.”
Read Our 2022 Research Annual Report

ABOUT THE STUDY
This research was supported by several grants from the NIH: U18 EB021780 (JMW, MAH), U19 AI116491 (JMW), P01 HD093363 (JMW), UG3 DK119982 (JMW), U01 DK103117 (MAH), 1F31DK118823-01 (AKE), NIEHS 5T32-ES007250-29 (DOK), the Shipley Foundation (JMW), and the Allen Foundation (JMW). This study also received support from the Digestive Disease Research Center (P30 DK078392).
| Original title: | Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells |
| Published in: | Cell Stem Cell |
| Publish date: | Jan. 6, 2022 |
Research By

Research in the Wells lab aims to uncover the molecular and cellular mechanisms by which gastrointestinal and endocrine organs form in the developing embryo.

As a pediatric surgeon scientist, Dr. Helmrath has established a large multidisciplinary team dedicated to clinical, translational, and basic science research focused on human diseases.

Our goal to elucidate the molecular mechanisms controlling the embryonic development of digestive and respiratory organs. We use a combination of Xenopus and mouse animal models, human pluripotent stem cells and cutting-edge genomics to investigate the underlying gene regulatory networks of organogenesis.





