New Tests May Help Diagnose Rare Subset of Eosinophilic Disorders
Research By: Tetsuo Shoda, MD, PhD | Marc Rothenberg, MD, PhD
Post Date: January 6, 2020 | Publish Date: January 2020
Achievement a product of nine centers cooperating as part of the Rare Diseases Clinical Research Network
As a group, eosinophilic gastrointestinal disorders (EGIDs) are rare and often hard to diagnose. The most common form—eosinophilic esophagitis (EoE)—affects an estimated 158,000 of more than 320 million people in the United States. Other forms, such as eosinophilic gastritis (EG), are much less common, which makes them even more difficult to accurately diagnose.
Now, a new study in the Journal of Allergy and Clinical Immunology, led by scientists at Cincinnati Children’s and based on data from a nine-center research consortium, introduces new tissue and blood testing platforms that may make it easier for specialists to pinpoint EG.
What is EG?
EG is a chronic disorder marked by gastric eosinophilia and other clinical symptoms.
The eosinophil is a specialized white blood cell found in several tissue types with varied functions in the immune system. Eosinophilic functions include movement to inflamed areas, trapping substances, killing cells, and anti-parasitic and bactericidal activity. They are implicated in numerous inflammatory processes, especially allergic disorders.
Chronic activation of eosinophils in tissues can cause disease. Patients with EGIDs have excessive eosinophilia in segments of the gastrointestinal tract.
In EG, having too many eosinophils in the stomach causes pain and inflammation. When the body wants to attack a substance, such as an allergy-triggering food or airborne allergen, eosinophils respond by moving into the area and releasing a variety of toxins. However, when the body produces too many eosinophils, they can cause chronic inflammation resulting in tissue damage.
There is no cure yet for EGIDs. Diagnosis and disease monitoring typically requires repeated endoscopies to collect sample biopsies. First-line treatment focuses on eliminating the foods causing the allergic reaction, which often results in severely restricted or formula-based diets.
Diagnosis, treatment and understanding of eosinophilic esophagitis (EoE), the most common EGID, has improved greatly in the last decade. However, making similar advances in EG has been more challenging.
Patients with EG often experience a long journey to correct diagnosis and effective treatment. For years, there have been no standardized diagnostic criteria, and little has been known about the causes of EG. These difficulties are largely due to EG being very rare and most patients with EG also having other EGIDs.
Only 1 in 5,500 patients have an EGID that is not EoE, and even fewer have EG by itself. With fewer individuals to treat and study, we have fewer opportunities to learn more about this condition and draw conclusions.
Consortium Steps In
The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR), a research team within the Rare Diseases Clinical Research Network, is overcoming these challenges. CEGIR is recruiting more people to participate in research, allowing sample sizes with sufficient power to make statistically meaningful observations.
The new study, led by first author Tetsuo Shoda, MD, PhD, and senior author Marc Rothenberg, MD, PhD, builds upon earlier work and the histologic characterization of EG in 2014 (Caldwell et al. JACI 2014). Shoda and colleagues examined gastric biopsy and blood samples from 185 individuals from nine CEGIR sites to develop improved diagnostic platforms for EG.
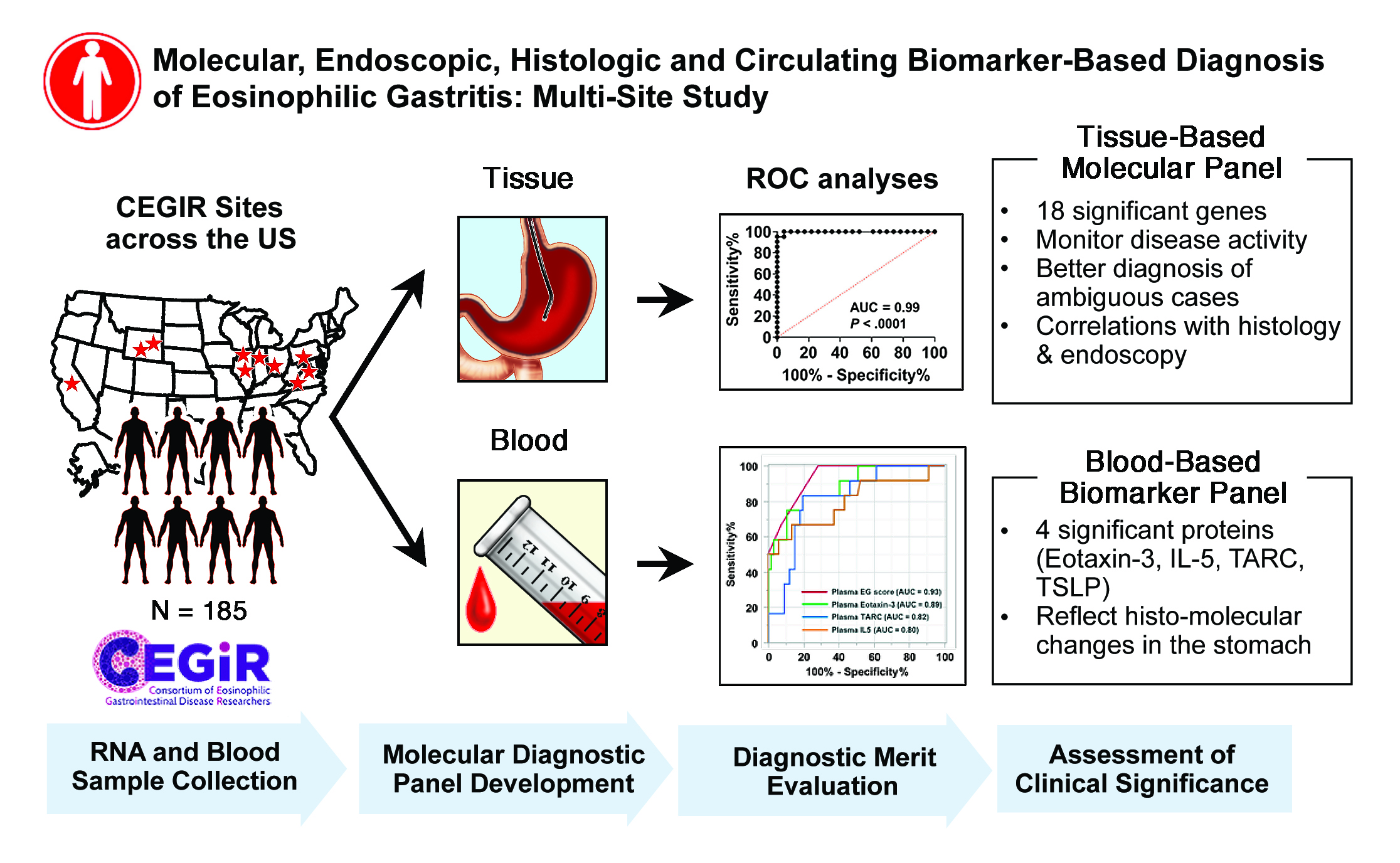
Data Sharing Produces Results
The biopsy and blood samples allowed the research team to develop a molecular profile that showed robust associations with histologic and endoscopic features. Plasma eotaxin-3 levels were strongly associated with gastric CCL26 expression, making this relationship the strongest single tissue and circulating disease biomarker.
What’s next?
These new tissue- and blood-based platforms for assessing EG were made possible by collaboration among CEGIR members. Further work is required before launching a prospective clinical trial to validate these platforms, explore their feasibility in various clinical settings, and ultimately optimize the platforms for disease stratification.
Learn more about EoE research and treatment at Cincinnati Children’s
Funding Notes
CEGIR (U54 AI117804) is a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. This consortium is funded through a collaboration between the NCATS, the NIAID and the NIDDK.
—Article written by Shawna Hottinger, MS, ELS, Division of Allergy and Immunology
| Original title: | Molecular, Endoscopic, Histologic and Circulating Biomarker-Based Diagnosis of Eosinophilic Gastritis: Multi-Site Study |
| Published in: | Journal of Allergy and Clinical Immunology |
| Publish date: | January 2020 |
Research By









