New ‘Atlas’ of Human Heart Valve Development May Guide Next-Gen Therapies
Research By: Mingxia Gu, MD, PhD | Yifei Miao, MBBS, PhD
Post Date: July 29, 2024 | Publish Date: July 24, 2024
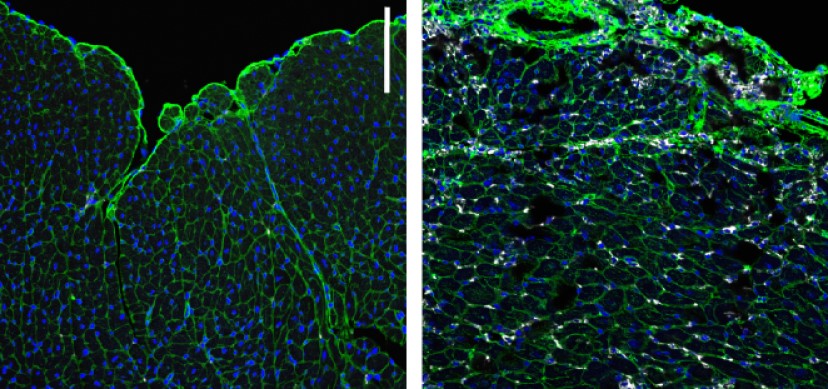
Study led by experts at Cincinnati Children’s sheds light at the cellular level on how heart valve tissues form. Findings eventually may improve survival odds for newborns with heart valve defects.
Imagine being able to both detect and treat potentially fatal heart valve defects months before a baby is born.
That day remains years away but has moved considerably closer to reality now that scientists have compiled an advanced “atlas” of human heart valve formation to guide future research. The new findings were published July 24, 2024, in Nature Cardiovascular Research.
Emerging blueprint for replacement valves that can grow
Valves are tiny but critical heart structures. In adults, surgeons have performed successful artificial heart valve replacements for many years. Some newborns also can be rescued with non-living valve transplants. However, these valves lack human cells and thus cannot form a fully normal valve structure. Additionally, they do not grow with the child and often require multiple high-risk replacement surgeries.
In hopes of improving outcomes, scientists have been hunting for ways to produce heart replacement valves that contain relevant valvular cell types and can grow with the patient.
“Engineered human heart valves with the appropriate cell types for transplantation would avoid repeated surgeries in babies and address the donor valve shortage,” says corresponding author Mingxia Gu, MD, PhD, an expert in molecular and cardiovascular biology at Cincinnati Children’s. “Now, we’ve achieved an unprecedented understanding of the cellular composition of human heart valves and how fetal heart valve tissue changes over time. This is a crucial first step for future human valve engineering.”
How do heart valve leaflets form?
A healthy human heart features four valves that help guide blood as it pumps through the heart’s chambers. Each valve has two or three thin and fragile-seeming leaflets that open and close more than 2.5 billion times across a lifetime.
During embryonic development, the types of cells forming those leaflets completely change. At 14 weeks gestation, the immature leaflets are made primarily from complex sugars called glycosaminoglycans. But by 36 weeks, the leaflets have morphed into triple-layered tissues complete with flexible elastin and stiff collagen fibers.
When this transformation–called remodeling–does not occur properly, the malformed valves can lead to severe and potentially fatal heart defects.
Heart valve birth defects are rare. The most common defect, called bicuspid aortic valve, is found in an estimated 0.5% to 2% of the general population. This condition occurs when two of the normal three leaflets of the aortic valve are fused into one. Those affected need life-long monitoring and often need valve replacement surgery at some point in their lives.
Other conditions that can involve defective heart valves include valve prolapse in Marfan syndrome, pulmonary valve stenosis in tetralogy of Fallot, and aortic valve atresia in hypoplastic left heart syndrome.
Unexpected role played by the gene APOE
The Cincinnati Children’s research team performed a deep study at single-cell resolution of healthy and defective human heart valves. The team documented in unprecedented detail how various valvular cell types communicate with each other to promote tissue formation.
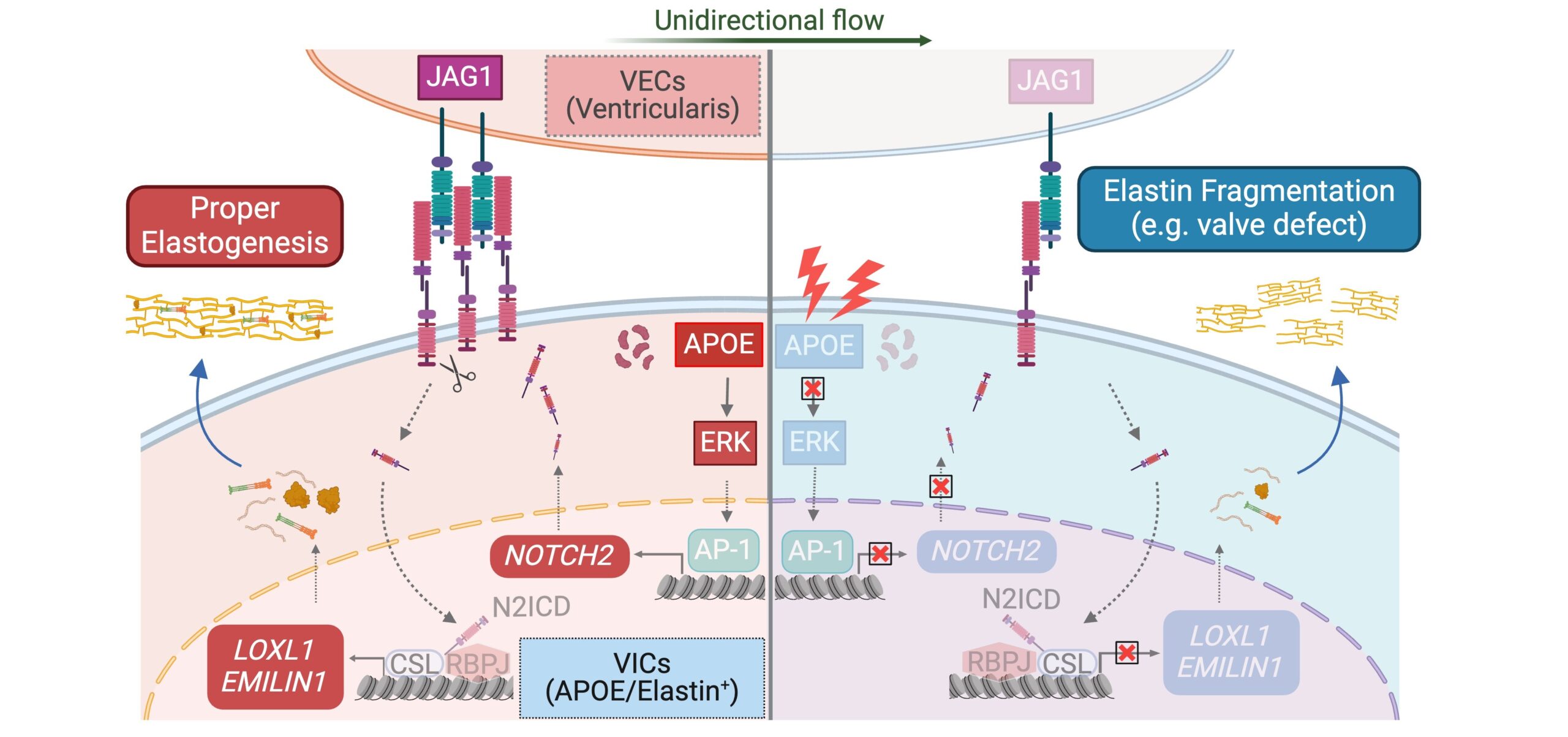
Among the findings, the team discovered a valvular cell type with enriched expression of the gene APOE. This gene needs to work in concert with another gene called NOTCH2 to produce the elastin fibers essential for heart valve function. The involvement of APOE was surprising, as it had previously been known only for its role in atherosclerosis and Alzheimer’s disease.
The paper further describes signaling “crosstalk” among key genes as they instruct developing cells to form the heart valve tissues. This process continues throughout fetal development and continues during infancy and childhood until the heart reaches its full adult size.
“The finding of APOE as the top regulator in modulating elastin formation during early valve formation could yield a new angle for clinicians to understand heart valve underdevelopment,” says co-corresponding author Yifei Miao, MBBS, PhD.
How can these findings help people with heart valve defects?
Several more years of study will be needed to translate these new findings into potential therapies. Understanding which genes are crucial for the development of specific valvular cell subtypes during human valve formation—and which are dysregulated in congenital valve diseases—will provide vital clues for developing gene or cell therapies for affected babies.
One exciting avenue for future research is using this new atlas to engineer a “human valve” for valve replacement therapy.
“This atlas provides insights into the similarities and differences among the four human valves, enabling more precise valve engineering and deep understanding of the valve-specific phenotypes in patients,” Gu says. “The newly discovered APOE+ valve cell type could be crucial for seeding onto an engineered valve scaffold to mediate elastin fiber formation, essential for valve remodeling and function.”
About the study
Cincinnati Children’s co-authors also included Ziyi Liu, MD, Yu Liu, PhD, Zhiyun Yu, PhD, Cheng Tan, MD, Nicole Pek, BSc, Anna O’Donnell, BS, MBA, Minzhe Guo, PhD, Katherine Yutzey, PhD, and David Winlaw, MBBS, MD, (now at Lurie Children’s Hospital).
Co-authors also included experts with the Stanford School of Medicine, the University of Michigan, the University of Washington, and the Icahn School of Medicine at Mount Sinai, New York.
Funding sources for this study include support from Additional Ventures (1019125), an Endowed Scholar Award from Cincinnati Children’s, two grants from the Chan Zuckerberg Initiative (CZF2019-002440 and CZF2021-237566), and two American Heart Association Predoctoral Fellowship grants (1013861 and 906513).
This research was further supported by the Cincinnati Children’s Heart Institute Biorepository, the Discover Together Biobank, the Division of Pulmonary Biology, and core research facilities in Pathology and Confocal Imaging.
| Original title: | APOE–NOTCH axis governs elastogenesis during human cardiac valve remodeling |
| Published in: | Nature Cardiovascular Research |
| Publish date: | July 24, 2024 |
Research By


In my research lab, my team and I attempt to identify the vasculature irregularities in congenital diseases, including single ventricle disease, valve disease and pulmonary hypertension