Location, Location, Location: A New Paradigm for Kidney Fibrosis
Research By: Prasad Devarajan, MD
Post Date: January 8, 2024 | Publish Date: Jan. 3, 2024
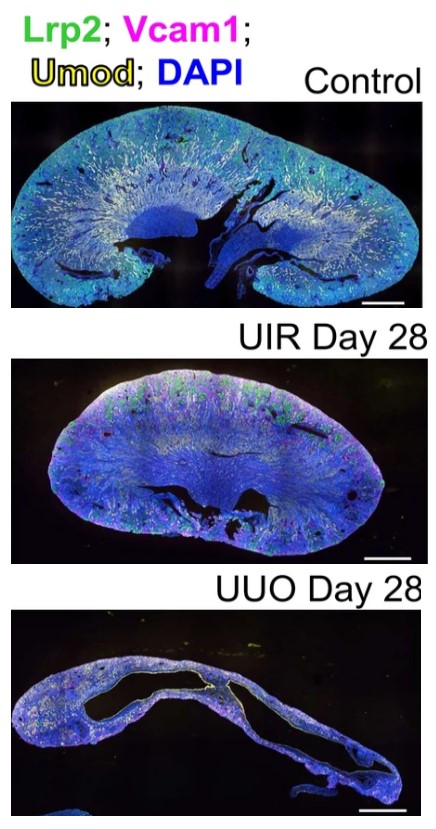
We believe that our findings represent a complete redirection of the landscape of persistent kidney injury away from the proximal tubule to the distal tubule location.
Acute kidney injury (AKI) is a devastating disease process that affects 20-30% of critically ill children and adults globally, with high rates of short-term morbidity and mortality. In most cases, AKI is a consequence of other serious systemic illnesses or other organ failures, and the kidney is an innocent bystander. Importantly, the burden of AKI extends well beyond the acute setting, with increased risk for progression to chronic kidney disease (CKD), end-stage kidney disease requiring dialysis or transplant, and long-term mortality.
Mechanistic studies thus far have been limited to the proximal kidney tubule; the nephron segment located adjacent to the filtering units of the kidney where much of the initial injury occurs. However, therapeutic targeting of the proinflammatory and profibrotic pathways in the proximal tubule has failed to improve the outcomes. The response to injury located at the downstream distal tubule segment, and the identity of cells that contribute to the chronic profibrotic changes, have hitherto been largely unrecognized.
Were we barking up the wrong tree? To answer this question in an agnostic and unbiased fashion, we set up two clinically relevant mouse models of AKI that reliably resulted in fibrotic CKD. The goal of the study was to unravel novel molecular and cellular mechanisms of advanced fibrotic kidney disease in all kidney locations.
We found that both animal models generated three novel clusters of previously unknown fibroblastic cell types that are likely to contribute to new profibrotic pathways via enhanced epithelium-to-fibrotic cell crosstalk. Unexpectedly, we also identified several pockets of remnant tubule epithelial cells that had failed to undergo regeneration and repair.
Most unexpectedly, these damaged tubule cells were identified to be primarily derived from the distal tubule location. Moreover, we documented that these cells from the distal tubule dramatically upregulated the expression of several genes normally seen only during early kidney development.
We believe that our findings represent a major paradigm shift, and a complete redirection of the landscape of persistent kidney injury away from the proximal tubule to the distal tubule location. These distal tubule cells are making a valiant attempt to repair themselves, by turning on regenerative genes and proteins that have been dormant since early development in utero. Failure of distal tubule repair is leading to the generation and amplification of novel fibroblastic cell clusters and profibrotic pathways.
What needs to happen next for this scientific advance to be further validated and ultimately translated into improved clinical care? First, we will need to identify the specific profibrotic pathways activated by the novel fibroblast clusters, and test whether inhibiting those mechanisms can prevent kidney disease progression. Second, we will need to enhance the identified regenerative response (genetically and pharmacologically) and tilt the balance from injury to repair in the distal tubule segments. It is our hope that we can derive enduring benefit from the kidney regeneration tools that we are all endowed with before birth to prevent progression of kidney disease at all stages of life.
-Post authored by Prasad Devarajan, MD
Co-authors on this study include first author Valeria Rudman-Melnick, Qing Ma, and Saagar Chokshi, all with the Division of Nephrology and Hypertension; Mike Adam, Kaitlynn Stowers, Andrew Potter, J. Matthew Kofron and S. Steven Potter, all with the Division of Developmental Biology; Davy Vanhoutte, Division of Molecular Cardiovascular Biology; Diana Lindquist, Department of Radiology and Medical Imaging; Michelle Nieman, UC Department of Pharmacology and Systems Physiology; Iñigo Valiente-Alandi, with San Francisco-based Cytokinetics; and Eunah Chung and Joo-Seop Park, both at Northwestern University.
| Original title: | Single-cell sequencing dissects the transcriptional identity of activated fibroblasts and identifies novel persistent distal tubular injury patterns in kidney fibrosis |
| Published in: | Scientific Reports |
| Publish date: | Jan. 3, 2024 |
Research By

Our research focuses on three areas: Investigating the mechanisms, biomarkers and novel therapies for acute kidney injury; studying the pathogenesis and biomarkers for focal segmental glomerulosclerosis; and identifying lupus nephritis molecular pathways and biomarkers.