Investigating Clinical Research Participation Among Children
Research By: Richard Ittenbach, PhD
Post Date: December 20, 2021 | Publish Date: Sept. 6, 2021
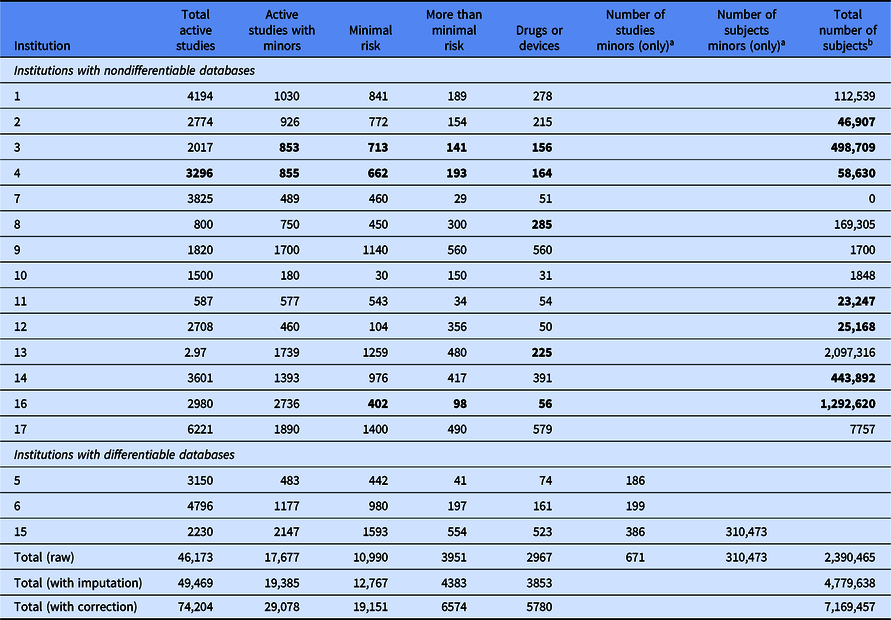
Most institutions were able to provide the number of approved studies involving minors and total numbers of studies involving drugs or medical devices, but few could report how many children were participating in the studies.
Written by Richard Ittenbach, PhD, and Jeremy Corsmo, MPH
How many children are participating in clinical research today? This knowledge is vital to understanding new discoveries, treatments, and diseases in children.
However, there is a significant gap in the scientific, peer-reviewed literature regarding national estimates of children’s inclusion in clinical research. Without this knowledge, it is nearly impossible to determine whether this critical need is being met.
In a study published Sept. 6, 2021, in the Journal of Clinical and Translational Science, we worked with our colleagues Alison Kissling, MLIS, and Arnold Strauss, MD, to survey IRB administrators at leading pediatric medical centers regarding studies with minors. Using data provided by the NIH, we identified a sample of the top institutions receiving the most NIH funding for pediatric-focused research in FY2018.
Of the estimated 74,204 active studies, 39 percent included minors, and 23 percent were “more than minimal risk.” Altogether, minors accounted for 0.7–2.87 million research subjects.
We found that most institutions were able to report overall summary numbers, such as total numbers of approved studies, total numbers of studies involving drugs or medical devices, or even total numbers of studies that have the potential to enroll some children. However, what was surprising is that when asked to report specifically the total number of children enrolled in all studies active at the institution, almost none of the responding institutions were able to provide that number. Only three of the 17 respondents appeared to have the capability of distinguishing between minors and adults in enrollment data for studies that included both—and of those three, only one was able to actually report a value.
Although we do not believe that this study suggests a regulatory deficiency at any of the participating institutions, it does suggest a major challenge in determining whether we, as a pediatric research community, are meeting our goal of ensuring that children are appropriately represented in research at the national level. Research institutions generally have substantial capacity to conduct complex research study-related data management and analysis activities, yet we suspect that this capacity and expertise is very rarely applied to curating and analyzing data that are available in their enterprise research administration and compliance systems.
Our hope is that this study will shed a light on a missed opportunity, among pediatric research institutions, to leverage improved data management capabilities in research administration. In many cases, such as estimating the total number of children participating in clinical research, there is significant untapped potential in this data that we are not taking advantage of. Knowing whether children are being adequately represented in research at the local and national levels will enhance our ability to advocate for new and expanded pediatric-focused research priorities, ultimately benefiting our local patients and community.
| Original title: | How many minors are participating in clinical research today? An estimate and important lessons learned |
| Published in: | Journal of Clinical and Translational Science |
| Publish date: | Sept. 6, 2021 |
Research By