Inclined to Predict Declines: A Tool for Informing Early Intervention for Cystic Fibrosis Patients
Research By: Assem Ziady, PhD | Rhonda Szczesniak, PhD | Cole Brokamp, PhD | John Paul Clancy, MD
Post Date: May 17, 2021 | Publish Date: December 2020
A person without a breathing problem will easily inhale and exhale tens of thousands of times a day, taking for granted this important signal of life. However, breathing isn’t as simple for children and adults with the inherited disease cystic fibrosis (CF).
CF is a life-limiting disease typically diagnosed by age 2. The build-up of fluids in the airways can cause a mucinous cough and repeated inflammation and infections of the lung, leading to respiratory failure.
Dramatic improvements in the last 50 years have greatly increased the quality of life and lifespan for people living with CF. However, these individuals still experience pulmonary function decline and exacerbation episodes (PEx), an acute worsening of respiratory symptoms.
Many patients experience multiple episodes a year, and each may require hospitalization and aggressive treatment with powerful intravenous drugs. In most cases, patients are not proactively treated without evidence of disease progression; the catch is that multiple PEx are lagging indicators for disease progression.
New tool could allow proactive intervention
Predictive and personalized measures of lung function decline, combined with biomarkers, could provide additional insights for clinicians to decide to initiate more frequent monitoring, or intervene with anti inflammatory, antibiotic or mycotic therapies as needed.
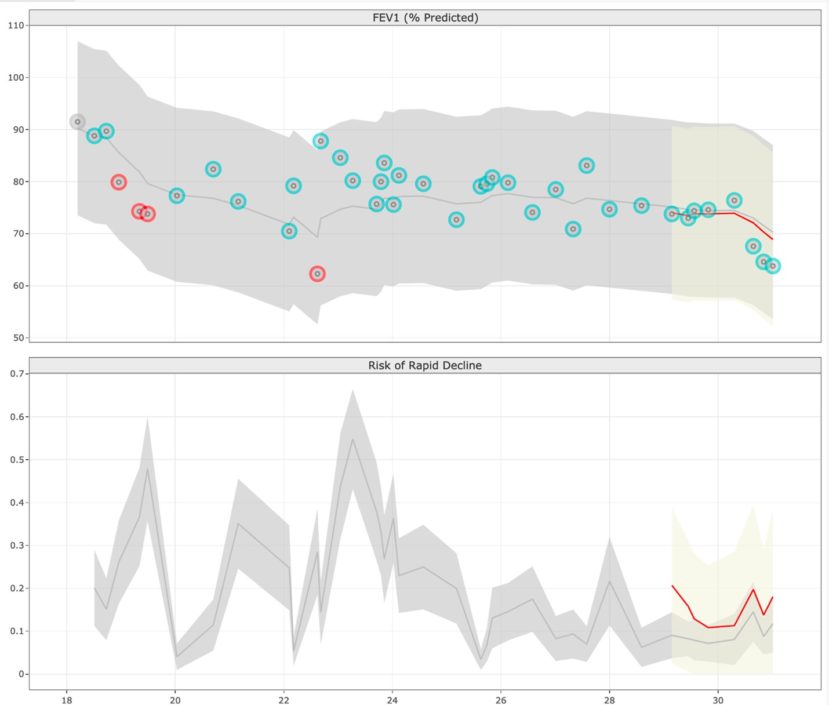
We have developed a functional data analysis-based prediction model that uses physiological and phenotypic variables in combination with a panel of novel prognostic blood biomarkers (identified through deep proteomic screens). Details were published in December 2020 in JMIR Medical Informatics.
Feasibility results show that the biomarker driven algorithm can predict lung function decline 3-6 months in advance of rapid lung decline with very good sensitivity and specificity. The test is optimized to identify patients that are at low risk of PEx, thereby allowing clinicians to more closely monitor other patients who may be at higher risk for PEx. Additionally, this model also has the potential to help accelerate CF clinical trials for potential therapeutics, with rapid outcome measures to determine the effectiveness of the proposed drug therapy in stemming disease progression.
Next Steps
The CF biomarker-informed lung function prediction test is being validated with more than 300 samples, courtesy of the Cystic Fibrosis Foundation’s biospecimen repository. The current prototype for predictions, utilizing demographic and clinical characteristics, is available at www.predictfev1.com.
Protein biomarkers also are being validated. Future activities include the clinical validation of the tool, which will involve collaborations with clinicians across the country.
Cincinnati Children’s Innovation Ventures has discussed the potential for this technology with early stage investors and there is strong interest in spinning out the technology as a startup to help deliver this test to CF care centers. The potential to help both children and adults living with CF is great.
About this Study
Funding sources for this technology include: the National Heart, Lung, and Blood Institute of the National Institutes of Health (K25 HL125954, HL116226, R01 HL141286, R01 HL142210, and R61 HL154105); Ohio Development Services (TECG2019-0159) and the Cincinnati Children’s Innovation Fund.
| Original title: | Cystic Fibrosis Point of Personalized Detection (CFPOPD): An Interactive Web Application |
| Published in: | JMIR Medical Informatics |
| Publish date: | December 2020 |
Research By