How ‘Pioneers’ Blaze the One Trail that Determines Cell Fate
Research By: Satoshi Matsui, PhD | Hee Woong Lim, PhD | Makiko Iwafuchi, PhD
Post Date: January 10, 2024 | Publish Date: Jan. 10, 2024
Developmental Biology | Top Scientific Achievement

Scientists at Cincinnati Children’s shed light on how a special set of transcription factors ride herd on developing tissues, not just by pointing cells to their proper place, but also by cutting off other ways of getting there. Findings offer significant implications for organ development, cancer research, and more.
One of the important breakthroughs that made it possible to program or reprogram cell fate more efficiently and with higher fidelity in a dish was discovering how to make use of a small set of molecular cowboys called pioneer transcription factors (TFs).
Every cell in our bodies has more than 200 transcription factors expressed inside, riding along the DNA helix instructing specific genes to activate and deactivate. During the early stages of fetal development, a small subset of “pioneer” TFs act inside our precursor cells driving them to make the mature cells that become parts of the spine, the heart, the liver, and so on.
Identifying the key pluripotent pioneer TFs helped researchers learn how to make induced pluripotent stem cells from any type of adult cells, which could then be instructed to make other types of cells, thus forming organoids that can mimic functional organ tissue. Furthermore, tissue-specific pioneer TFs can directly reprogram one adult cell type into a targeted cell type without passing through a pluripotent state.
Currently, there is considerable interest in producing therapeutic target cells from pioneer TF-mediated reprogramming of skin or other accessible somatic cells, as they hold great promise for personalized disease modeling and regenerative medicine. However, a major challenge is achieving sufficient gene repression of the original cell type, as inadequate repression often leads to the formation of “dead-end” cells or hybrid cells, thus limiting translational applications.
Now a study published online Jan. 10, 2024, in Molecular Cell, reveals important new details about how pioneer TFs do their important jobs.
“As we gain a more comprehensive understanding of the mechanisms underlying pioneer TF-mediated gene repression, it will greatly enhance the precise manipulation of cell fate in cellular programming and reprogramming,” says Makiko Iwafuchi, PhD, a member of the Division of Developmental Biology and the Center for Stem Cell & Organoid Medicine at Cincinnati Children’s.
The first author, Satoshi Matsui, PhD, and Iwafuchi collaborated with Hee-Woong Lim, PhD, a co-senior author and a member of the Division of Biomedical Informatics, on the study.
Until this study, most experts believed that pioneer TFs functioned primarily by activating genes to send cells toward their ultimate fates. However, scientists have learned that our genetic programming is packed with alternative pathways, which must be actively shut down.
So how does the body ensure that precursor cells consistently follow the correct path? It turns out that pioneer TFs do more than push cells in the desired direction. When functioning correctly, these cowboys ride ahead and cut off alternative paths so that developing cells keep developing as intended.
Who are these pioneers?
The Cincinnati Children’s team established a new CRISPR interference (CRISPRi) model to explore how the pioneer transcription factor FOXA controls human endoderm differentiation during liver development, and how the pioneer transcription factor OCT4 influences the behavior of pluripotent stem cells.
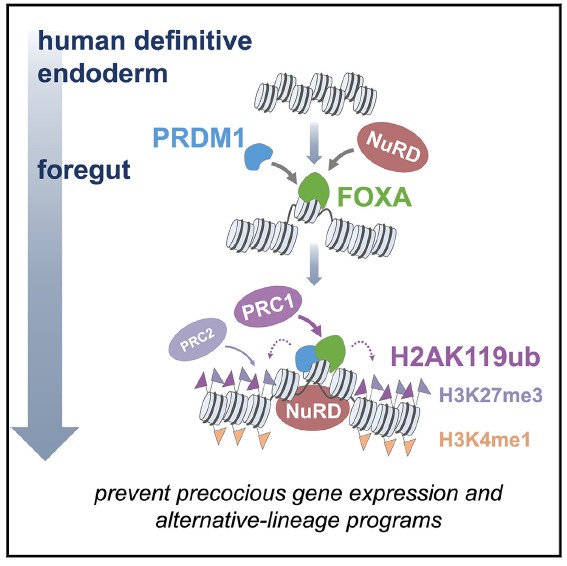
They found that when FOXA function was disrupted, cells followed multiple development pathways. But when FOXA functioned normally, the cells stayed on track. This allowed the team to conclude that FOXA prevents alternative lineage and precocious gene expression.
The team went on to show that FOXA gets help with repressing access to alternative pathways from another transcription factor called PRDM1 and other epigenetic repressors. Meanwhile in pluripotent cells, the pioneer transcription factor OCT4 performs a similar repression function by teaming up with a related transcription factor called PRDM14.
The central role of FOXA and its ability to repress development pathways were unexpected and critical findings that could influence future organoid and cell reprogramming studies, Iwafuchi says.
Finding a similar relationship occurring with OCT4 was an important further proof of concept because that transcription factor was already known to be among the pioneer TFs that enable reprogramming of somatic cells into pluripotent stem cells.
Next steps
Now that researchers have shown that two pioneer TFs coordinate with PRDM TFs to safeguard cell fate, it appears likely that other TFs have similar relationships. As these TF teams are identified, it may become possible to produce organoids and other engineered tissues in larger volumes with higher degrees of consistency and fidelity—steps that will be important for scaling up the technology.
About the study
In addition to Matsui, Iwafuchi, and Lim, Cincinnati Children’s co-authors included Marissa Granitto, Morgan Buckley, Katie Ludwig, Sandra Koigi, and Joseph Shiley, and Christopher Mayhew, all with the Division of Developmental Biology; and William Zacharias, Division of Pulmonary Biology.
Funding sources for this study include the National Institutes of Health (1R01GM143161), the Digestive Health Center (P30 DK078392), the Japan Society for the Promotion of Science Foundation, the Uehara Memorial Foundation, and the Cincinnati Children’s Research Foundation (Trustee Awards and Center for Pediatric Genomics Pilot Awards).
Explore the 2024 Research Annual Report
| Original title: | Pioneer and PRDM transcription factors coordinate bivalent epigenetic states to safeguard cell fate |
| Published in: | Molecular Cell |
| Publish date: | Jan. 10, 2024 |
Research By


In my research lab, I study fundamental mechanisms of gene transcriptional regulations in various contexts, such as metabolism, general cellular or tissue development, pathogenesis, and pharmacogenomics.

The long-term goal of the Iwafuchi lab is to reveal gene and chromatin regulatory principles underlying dramatic cell fate changing events that occur in embryonic development, tissue repair (physiological reprogramming), and diseases, and ultimately to utilize this knowledge to precisely engineer cell fates.