FDA Approval of HLH Drug Caps 15-Year Research Journey
Research By: Michael Jordan, MD
Post Date: July 1, 2019 | Publish Date: November 2018

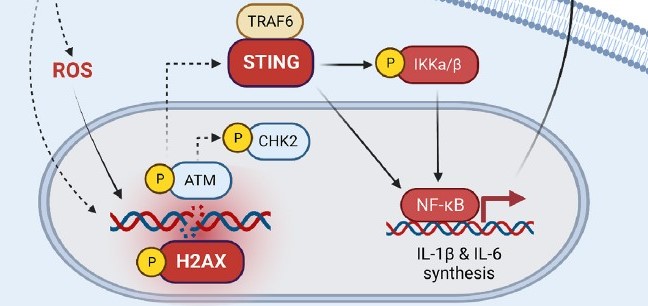
In 2004, Cincinnati Children’s physician-scientist Michael Jordan, MD, led a study in mice that identified what appeared to be molecular drivers of the mysterious and fatal immune disorder hemophagocytic lymphohistiocytosis (HLH).
That discovery led Jordan and a corps of parents, advocates and fellow researchers on a nearly 15-year journey to the first approved treatment specifically for HLH. The U.S. Food and Drug Administration gave the drug, Gamifant (emapalumab-IZsg), the green light in November, 2018.
Jordan calls the approval “amazing.”
“My goal for our work over all these years has been to use scientific exploration and practical medical approaches to make a difference in the lives of these children,” he says. “It’s been a privilege to help develop this idea from an unexpected laboratory discovery to an approved medicine.”
Jordan’s 2004 mouse study, published in the journal Blood, showed that elevated levels of the protein interferon gamma (IFNg) are essential to the HLH disease process. Gamifant, made by Sweden-based Sobi, specifically targets and blocks IFNg.
Reaching this point included patient families and advocacy groups working closely with Jordan and colleagues at Cincinnati Children’s HLH Center of Excellence. Fundraising efforts included more than $1 million raised in seven annual “700 Miles to Hope” marathon bicycle rides from Jackson, MS, to Cincinnati.
Research By