Early-Stage Study Suggests Potential Approach to Preventing AML
Research By: Daniel Starczynowski, PhD
Post Date: January 18, 2022 | Publish Date: Jan. 18, 2022
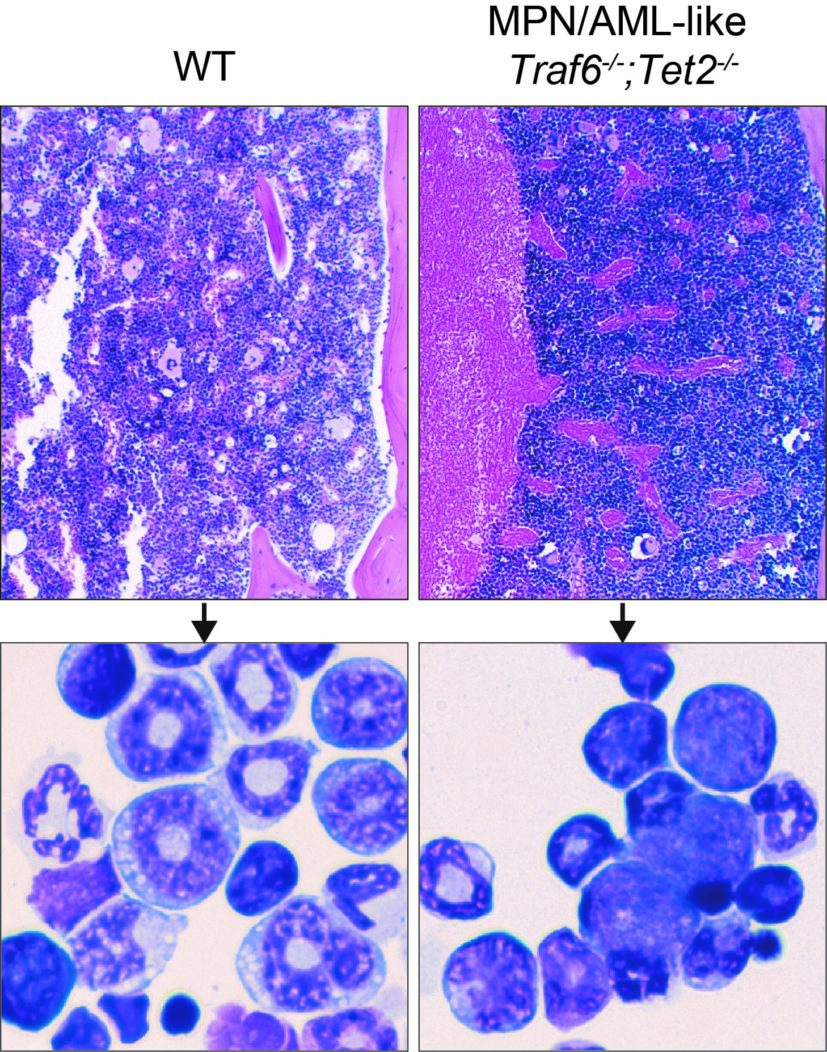
Experts from Cincinnati Children’s, NYU, and Japan discover, in mice, that the gene TRAF6 can block progression from pre-leukemia to full-blown disease
Acute myeloid leukemia (AML) is a form of blood and bone marrow cancer that strikes about 20,000 people in the U.S. a year. Symptoms can appear suddenly, prompting immediate intense chemotherapy, other targeted therapies and sometimes stem cell transplantation. While nearly 70% of children with AML survive at least five years, the five-year survival rate for adults lingers at a disappointing 26%.
Daniel Starczynowski, PhD, a cancer research expert at Cincinnati Children’s, along with research teams around the globe, has been hunting for years to find ways to improve those odds. Based on discoveries he published in 2019, the start-up company Kurome Therapeutics was launched to pursue new ways to prevent AML from adapting so easily to existing chemotherapies. Now, a new study in collaboration with Iannis Aifantis, PhD, and his team at NYU, published Jan. 18, 2022, in Cell Stem Cell, sheds even more light on the chain reactions that can lead from a state of pre-leukemia to full-blown disease.
What is the gene TRAF6?
A large number of scientists have described how people can acquire gene mutations in blood-forming cells during their lives that lack initial evidence of disease but make them increasingly likely to develop AML. These precursor conditions have been described as clonal hematopoiesis of indeterminant potential – “CHIP.” However, much has remained unknown about exactly how CHIP becomes full-blown leukemia.
The new study reports that the gene TRAF6 plays a crucial—and unexpected—cancer-regulating role amid a complex set of interactions between cellular mechanisms that govern blood cell production and innate immune-related pathways, including toll-like receptors (TLR). Too much TRAF6 activity can lead to certain types of cancer risk. Not enough TRAF6 activity clearly increases the risk of developing myeloid malignancies, such as myelodysplastic syndromes (MDS) and certain subtypes of AML. Having a balanced level of activity prevents the disease, and restoring that balance appears to improve survival once the disease appears—at least in mice.
“The role of TRAF6 has been investigated for its role in immune cell biology. However, more recently it has come to the forefront as a complex and key player in cancer. Some research indicates that it acts as a tumor suppressor, while others have reported that it can cause cancer,” Starczynowski says. “Our findings confirm that TRAF6 can, in fact, have opposite effects depending on the context of its environment. But we also found that its activity can be regulated through activation of certain toll-like receptors – evolutionarily conserved pathogen detectors in our immune systems that have been hijacked by the AML cell.”
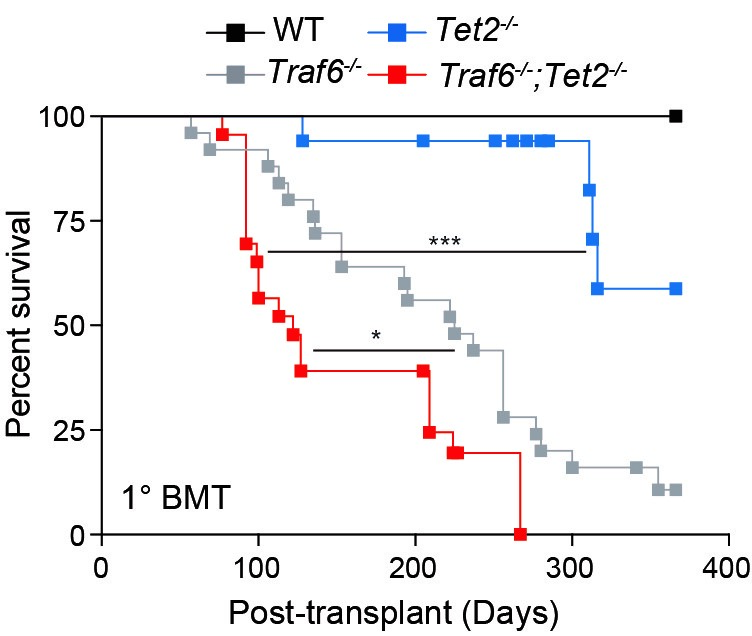
What does this discovery mean for people with MDS or AML?
The new information, based on an extensive series of mouse studies, remains in the early stages. Potential novel therapies and clinical trials based on these finding remain years away.
However, the research team has identified novel mechanisms underlying the stepwise progression of certain subtypes of AML, and in doing so may have zeroed in on a gene target that could bring the emerging field of inflammation-targeted therapies to bear against a notoriously hard-to-treat condition.
Next steps
“Our current studies primarily focused on mouse models, however more extensive work on human AML is warranted,” Starczynowski says. “Moreover, we are now launching into a series of experiments to help us understand the mechanistic basis for why TRAF6 can function both as a tumor suppressor and an oncogene. Through these studies, we hope to uncover novel therapeutic opportunities that will exploit the unique functions of TRAF6 in myeloid malignancies.”
View news coverage from WVXU
Learn more about Kurome Therapeutics
About this study
Funding sources for this study include the National Institutes of Health (R01CA216421, R01CA173636,
R01CA228135, R01CA242020, 1RO1CA266212, 1RO1HL159175, R35HL135787, R01DK102759, R01DK113639, L40HL143713, and R01CA190261), the Edward P. Evans MDS Foundation, the Leukemia and Lymphoma Society, the Uehara Memorial Foundation, the NY State Department of Health IDEA and IIRP programs, the Waksman Foundation of Japan, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, Cincinnati Children’s Hospital Research Foundation, Japan Society for the Promotion of Science, Cancer Free Kids, and a Pelotonia Fellowship.
Starczynowski serves on the scientific advisory board and has equity in Kurome Therapeutics. He also is a consultant for Kymera Therapeutics, Captor Therapeutics, and Tolero Therapeutics.
| Original title: | TRAF6 functions as a tumor suppressor in myeloid malignancies by directly targeting MYC oncogenic activity |
| Published in: | Cell Stem Cell |
| Publish date: | Jan. 18, 2022 |
Research By