Dendritic Cells Resist Cell Death to Prime Immune System’s Infection Response
Research By: Chandrashekhar Pasare, DVM, PhD
Post Date: May 5, 2020 | Publish Date: May 5, 2020
An important clue has emerged as to how the immune system mounts T cell responses against virulent pathogens, according to a study published online May 5, 2020 in Cell Reports.
When cells such as macrophages sense virulent bacteria like Salmonella, they die rapidly by an inflammatory process called pyroptosis. However, mounting a T cell response to combat intracellular bacterial infections is critical for bacterial clearance. Now research led by experts at Cincinnati Children’s reports that dendritic cells in the immune system protect themselves from pyroptosis-mediated death so that they can activate T cells against bacterial invasions.
These findings tease apart the complexities of cell-specific immune responses to infections and suggest that these differences can be exploited to design better vaccines and therapies against infectious diseases, says corresponding author Chandrashekhar Pasare, DVM, PhD, Division of Immunobiology.
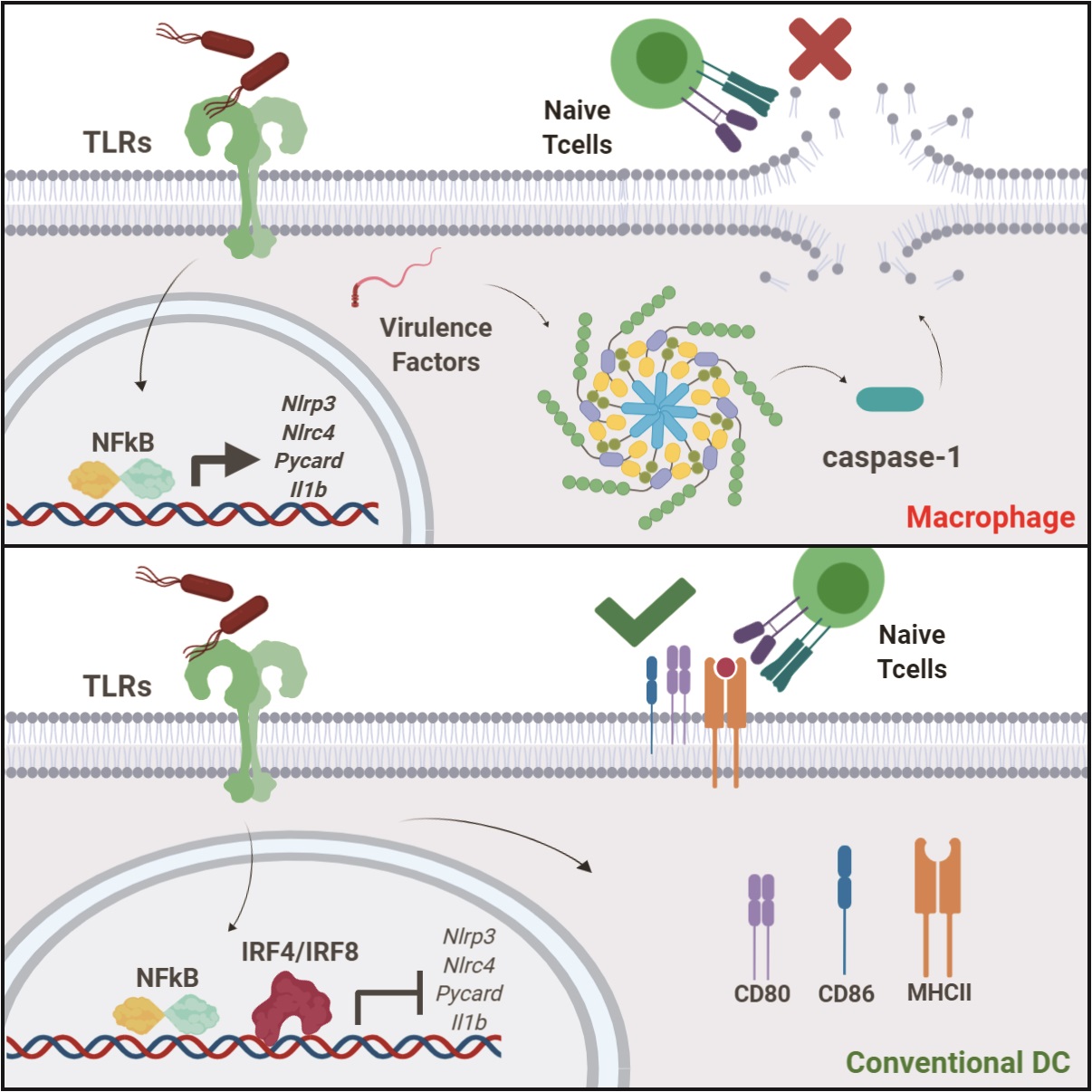
During the early course of a Salmonella infection when macrophages come into contact with the bacteria, they initiate a specialized immune response called inflammasome activation. This leads to secretion of several inflammatory proteins but also causes macrophages to die by pyroptosis. In fact, cell death occurs in less than an hour after coming into contact with such bacteria. This form of cell death actually benefits the host, as it expels the intracellular Salmonella so that it can be destroyed by other immune molecules such as antibodies and proteins of the complement cascade.
Dendritic cells (DCs)—a cell type closely related to macrophages—are necessary to relay important information about the invading pathogen to T cells, in order to mount a protective immune response. This process of T cell activation can take anywhere from 24 to 72 hours. This led the researchers to ponder how adaptive immunity is established against virulent pathogens if sensing of virulent pathogens leads to cell death. They began with the hypothesis that DCs might have evolved to resist inflammasome activation as that would be a detrimental immune response for long-term protection of the host.
“These findings have uncovered fundamental differences between two major cells of the innate immune system, macrophages and dendritic cells, and how they respond to virulent pathogens” the co-authors write.
Previous research by Pasare and colleagues has established that DCs shape adaptive immune responses depending on the nature of the pathogen.
In this study, they analyzed the differences between macrophages and DCs and found that DCs suppress inflammasome activation in response to virulent Salmonella infection. While DCs use other receptors to sense pathogens, they specifically block inflammasome activation to protect themselves from death so that they can survive to activate T cells. The researchers discovered that DCs use two key transcription factors known as IRF4 and IRF8 to prevent inflammasome activation. The study further analyzed DCs from mice lacking IRF8 expression and found them to undergo inflammasome-induced cell death and therefore resulted in defective T cell activation.
“This study provides fundamental understanding of how T cell responses are elicited against highly virulent bacteria by discovering key molecular details of suppression of inflammasome activation in DCs.” Pasare says. “More importantly, this study has implications for vaccination strategies, some of which use inflammasome activating ligands.”
In addition to Pasare, co-authors from Cincinnati Children’s include first author Margaret McDaniel, BSc, Division of Immunobiology; Leah Kottyan, PhD, Center for Autoimmune Genomics and Etiology; and Harinder Singh, PhD, previously a member of Division of Immunobiology and now at the University of Pittsburgh.
| Original title: | Suppression of Inflammasome Activation by IRF8 and IRF4 in cDCs Is Critical for T Cell Priming |
| Published in: | Cell Reports |
| Publish date: | May 5, 2020 |
Research By