COVID-19 Vaccine Development and Implementation
Post Date: February 1, 2021 | Publish Date:
Seminar Recap: Even as the number of vaccines grows, masking and distancing precautions remain necessary.
On Jan. 27, 2021, Robert W. Frenck Jr., MD, discussed the development of the current leading COVID-19 vaccine candidates, reviewed data from the recent clinical trials, and described potential issues with vaccine delivery implementation.
More than 270 people tuned in to hear Frenck’s presentation, “COVID-19 vaccine development and implementation: the here and now” during the second installment of the Current Topics in COVID-19 Research Seminar Series (click here to watch the recording). Hosted by the Cincinnati Children’s Divisions of Infectious Diseases and Biostatistics & Epidemiology, these free virtual seminars feature discussions with cross-disciplinary research professionals on the latest COVID-19 information and science.
Frenck is the director of the Cincinnati Children’s Vaccine Research Center and a renowned expert in vaccine development. He has been at the forefront of multiple COVID-19 vaccine trials taking place at Cincinnati Children’s, including the Pfizer multiphase trials in adults and adolescents as well as the AstraZeneca trial in adults.
THE IDEAL COVID-19 VACCINE
Frenck described the World Health Organization’s (WHO) ideal vaccine for COVID-19 and referenced this document, which helped guide and prioritize the development of these vaccines.
The first priority for this vaccine and any other is safety. “The only way you have no risk in research is that you don’t do research,” Frenck said. “Whenever you start to conduct research, there will always be risk, but we want to maximize our benefit and minimize our risk.”
The WHO would prefer a vaccine to have:
- A favorable benefit-to-risk ratio in the context of observed vaccine efficacy
- Only mild, transient adverse events (AEs) and no serious AEs
- To be well tolerated in individuals of all ages, especially elderly individuals who have the highest risk of mortality from COVID-19
- To meet an efficacy benchmark of 70% or higher
- To be capable of storage at warmer temperatures
- To be effective with a single dose, but two-dose vaccines are acceptable
“The best would be if you could put something on the shelf at room temperature,” said Frenck. “Vaccines that have to be stored at colder temperatures are less preferable.”
Frenck stated that any route of administration is acceptable as long as the vaccine is safe and effective. However, reflecting on his past experience working overseas, he mentioned that administration involving needles is inherently more complicated.
“If you have a vaccine that is oral or internasal, that will be more acceptable in many countries,” he said.
Lastly, there is a need for rapid scale-up of vaccine production during outbreak conditions that would allow for broad use.
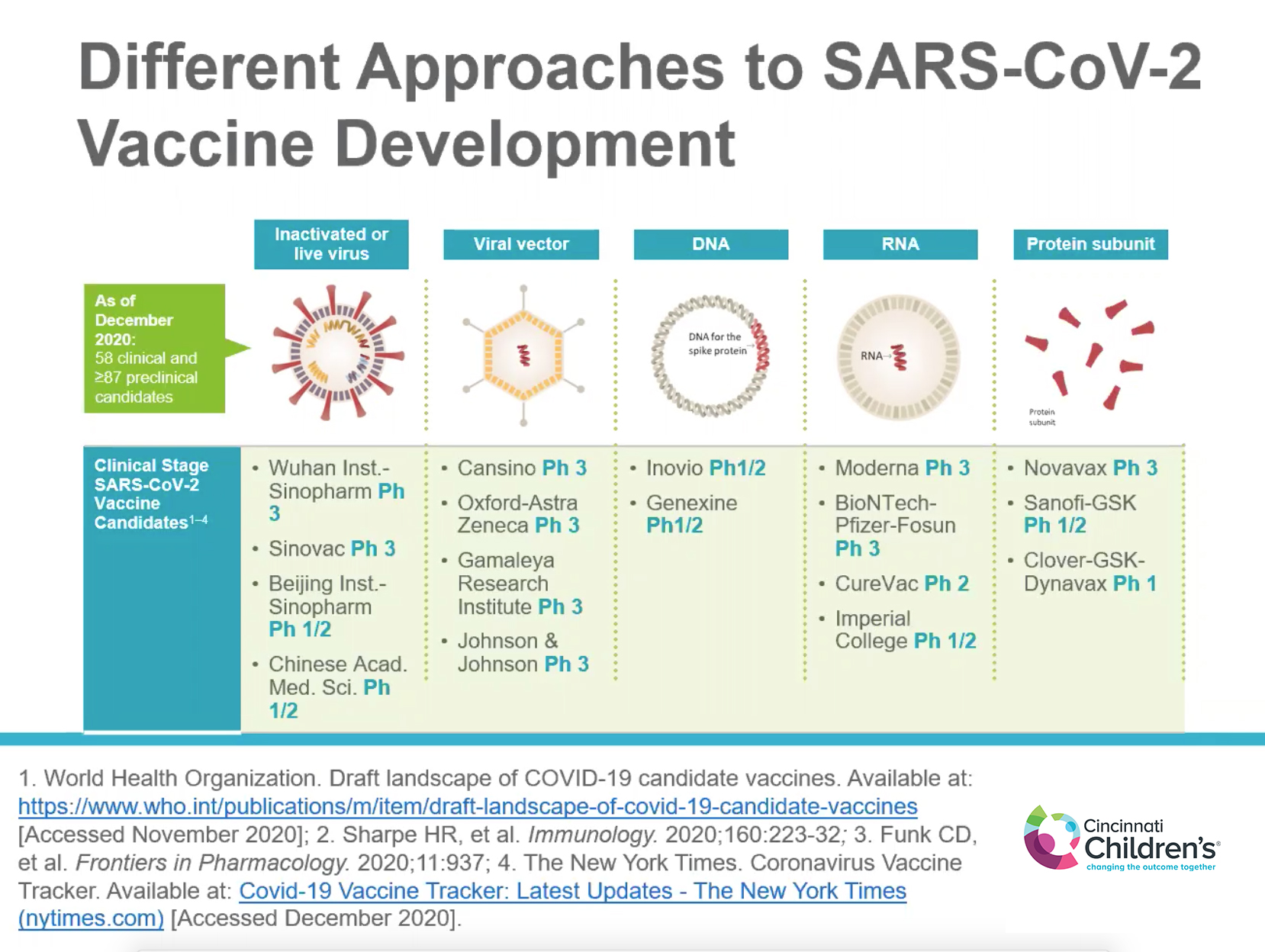
THE HERE AND NOW
Frenck continued by describing the landscape of COVID-19 vaccine candidates and each of the different development approaches, including live-attenuated or inactivated pathogen platforms, viral vector (AstraZeneca and Johnson & Johnson vaccines), DNA, RNA (Moderna and Pfizer vaccines), and protein subunit.
“The mRNA COVID-19 vaccines are now studied in several large-scale Phase 1, 2, and 3 clinical trials and have been generally well tolerated,” he said.
Fatigue and headache were two of the common adverse events reported in the vaccine clinical trials.
“There was much more fatigue reported in the younger group (18 to 55 years) rather than the older group (56 years nd up),” he noted.
Frenck said older participants likely also felt fatigue, but might have attributed their experience to daily life rather than a vaccine side effect.
For the AstraZeneca and Johnson & Johnson COVID-19 viral vector vaccines, Frenck confirmed that they cannot replicate in our cells because they are replication-deficient mutants of adenovirus. “Their mission is to bring the gene of the spiked protein into the cell and then release,” Frenck said.
He then went on to describe the complexities of human immunity to adenoviruses.
“If you administer an adenoviral vaccine, one of the concerns is that the immunity could be neutralized by our preexisting antibodies before it even gets to the cell,” he explained. “That’s why the AstraZeneca vaccine uses a chimpanzee adenovirus because almost all humans do not have preexisting antibodies to this.”
COVID-19 VACCINE MISCONCEPTIONS
Frenck cleared up common COVID-19 vaccine misconceptions by confirming that they do not make you infertile, modify your DNA in any way, cause you to test positive on PCR tests, give you the COVID-19 disease, or protect you against flu.
Some seminar viewers were also surprised to learn that COVID-19 vaccines are recommended even if you have previously been infected with the virus, as this may boost immunity to higher levels than natural immunity. Additionally, the previous COVID-19 infection may not have been sufficient to stimulate a good immune response.
“That’s why we are advocating for everyone to get the vaccine,” Frenck noted.
LOOKING TO THE FUTURE
Frenck reminded viewers that COVID-19 is not going away and these vaccines currently do not give a “license” to the recipient to change their behaviors, including masking and social distancing.
More COVID-19 vaccines are likely to be available in the near future in the U.S., including the Janssen and AstraZeneca vaccines.
“We have two vaccines available now,” he said, referring to the Emergency Use Authorization Pfizer and Moderna vaccines. “By the end of February, we should have two more.”
Pediatric vaccine trials are currently underway for Pfizer and Moderna. Pregnant women vaccine trials, stability studies, and second-generation vaccines are also being planned for the near future.
Watch a recording of the entire seminar.
WHAT’S NEXT?
Next up in the Current Topics in COVID-19 Research Seminar Series: Justin Lessler, associate professor at the Johns Hopkins University Bloomberg School of Public Health. Lessler will present “Understanding COVID-19 transmission: from households to populations.”
Lessler will describe why the key to controlling SARS-CoV-2 is understanding how the virus moves from one person to another, and what actions we can all take to stop this from happening. He will examine how to merge evidence from contact tracing studies and population level trends to gain a full picture of viral transmission.
Join this free virtual seminar at 2 pm (ET) Feb. 10, 2021, via Zoom.