Cheng, Frenck Speak to Congressional Staffers About Vaccine Trials for Children
Post Date: February 16, 2021 | Publish Date:

Tina Cheng, MD, MPH, chief medical officer, and director of the Cincinnati Children’s Research Foundation, and Robert Frenck, MD, director, Vaccine Research Center, presented to U.S. Congressional staffers on the topic “Developing COVID-19 Vaccines: Lessons from the Trial Frontlines & Actions Needed to Evaluate in Children.”
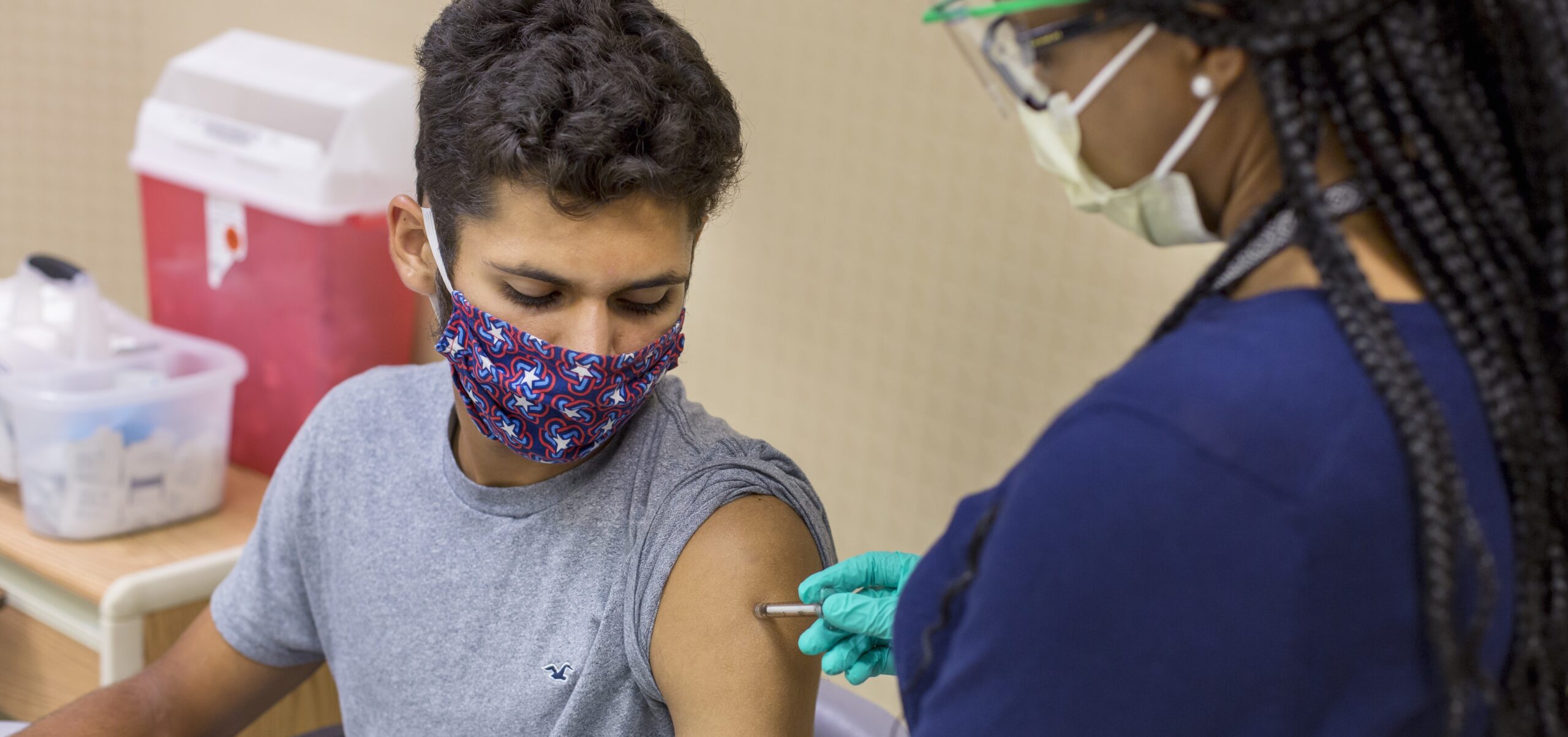
As one of 10 National Institute of Allergy and Infectious Diseases Vaccine Treatment and Evaluation Units and the only one located at a children’s hospital, Cincinnati Children’s has been a site for COVID-19 vaccine trials. Over the past several months, we’ve enrolled more than 1,000 study participants, including teenagers aged 12 and older.
Frenck, the lead investigator of the trial, discussed Cincinnati Children’s experience as a trial site. He also explained what actions will be needed to evaluate the shots in younger populations with the goal of producing vaccines that are safe and effective for children.
Cheng suggested protocols and public policies to support equitable access to COVID-19 vaccines across minority populations.
The Feb. 12 briefing also featured the perspective of a teenage participant in the trial.