Can the Bugs in Our Guts Help Manage Inflammation?
Research By: Emily Eshleman, PhD | Theresa Alenghat, VMD, PhD
Post Date: January 30, 2024 | Publish Date: Jan. 30, 2024
Eye-opening research from experts at Cincinnati Children’s suggests it’s possible
Imagine a day when people can enjoy snacking on foods designed to “re-calibrate” their microbiomes—that vast population of friendly bacteria living in our intestines—in order to promote a healthy immune system and decrease infections or chronic inflammation.
Experts at Cincinnati Children’s have made a significant leap toward better understanding how these resident microbes instruct intestinal health in a far-reaching study published online Jan. 30, 2024, in the journal Immunity. The research, led by immunologists and microbiome experts, Theresa Alenghat, VMD, PhD and Emily Eshleman, PhD, reveals a novel way to control allergic types of immune responses by adjusting how our intestines produce tuft cells, a lesser-understood element of our immune system.
While many people know that our intestines are fundamental to absorbing nutrients from the food we eat, fewer people realize that the 22-foot tube curled into our abdomens also serves as the largest component of our immune defense against invading pathogens. Scientists have studied the intestine for many years, but even now they are learning surprising details about how it works.
“This study shows that the microbiota—and products made by the microbiota—can dynamically control immune responses in the gut,” Alenghat says.
The research examines a reaction called type 2 immunity that occurs with chronic inflammatory conditions such as allergy and asthma.
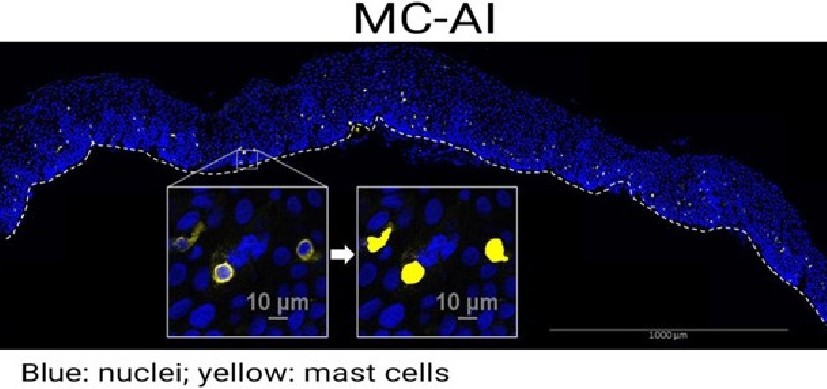
“We found that specific products made by bacteria in our gut can affect the power of type 2 reactions by controlling how many tuft cells form in the intestine’s mucosal lining,” Eshleman says.
Until now, it was not clear if nor how the gut microbiome impacts tuft cells. But now that key elements involved in this developmental pathway have been identified, these findings open doors to designing new diet and microbiome approaches for preventing or promoting type 2 inflammation.
A tale of fatty acids, organoids, stem cells—and worms
Studying what goes on in the intestine has long been a scientific challenge because the gut environment is incredibly dynamic.
In addition to all sorts of bacteria, fungi and viruses coming into the system, along with all the types of food and drink we consume, the intestine is constantly rebuilding itself. Our bodies continuously replace the entire epithelial lining from intestinal stem cells every two to five days. This means that the types of cells lining the intestine can often change, making them challenging to study in depth.
In this project, Alenghat and colleagues used a combination of mouse models and human intestinal organoids (tiny spheres of functional human tissue grown in a lab dish) to measure tuft cell responses in action. Their goals were to figure out what drives the process during health and in the presence of inflammatory triggers.
This is where worms enter the picture.
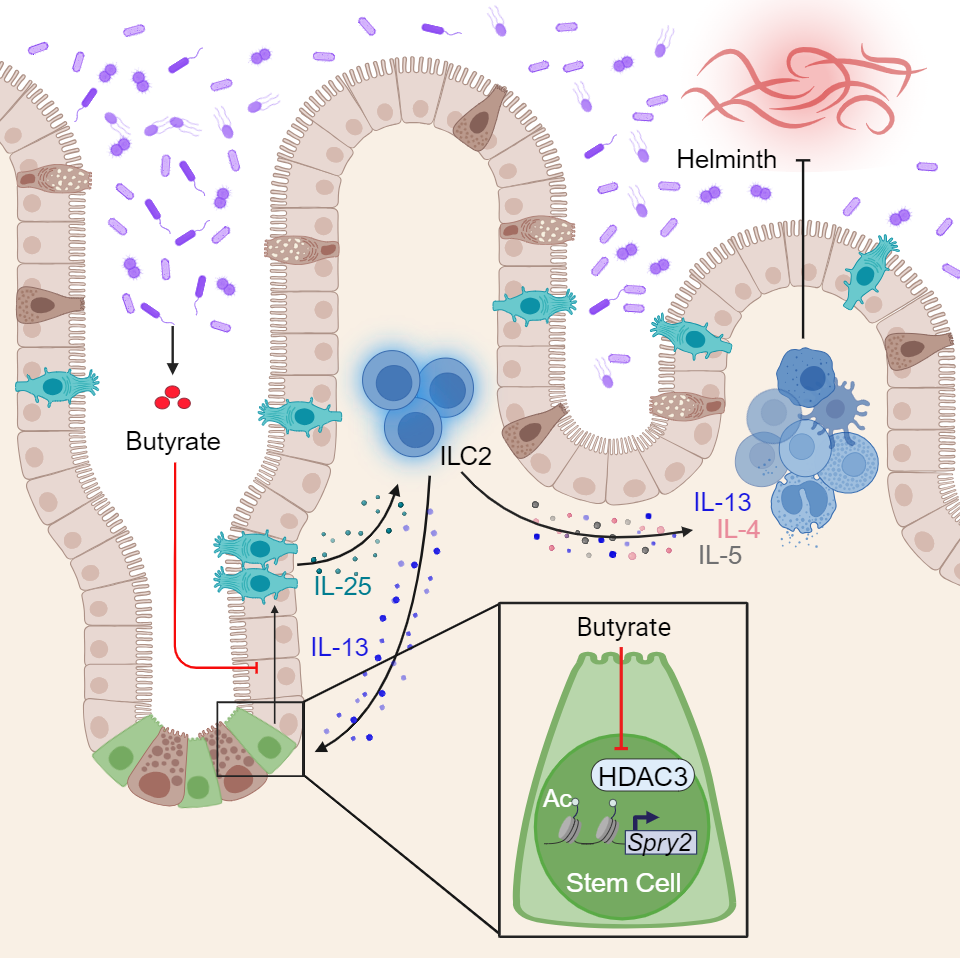
Millions of people worldwide pick up “helminth infections”, such as hookworms, roundworms and whipworms. It turns out that these parasites trigger similar type 2 immune reactions as a food allergy does. So by introducing worm infections to mice, researchers can study regulation of these immune responses in a controlled fashion without causing potentially dangerous allergic reactions in people.
What’s a tuft cell?
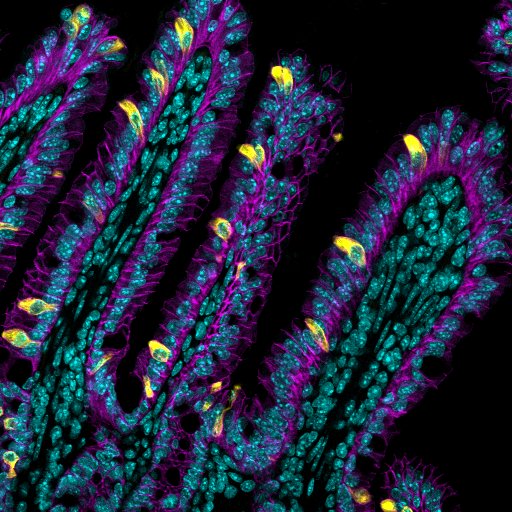
Scientists figured out that cells exist back in the 1600s. But tuft cells weren’t discovered until relatively recently—the 1950s. Their link to type 2 inflammation was made only within the last 10 years. These cells, so named because they are topped with fuzzy tufts of hair-like structures, emerge in widely variable, constantly changing numbers along the lining of the intestine.
Researchers hunting for cures for parasite infections learned that the intestine forms large numbers of tuft cells during worm infections and that tuft cells sustain an immune cycle that gets rid of the worms.
“This is one of many important reasons why we need a healthy intestinal immune system,” Alenghat says.
The Cincinnati Children’s team shed light on how the tuft cell production process works. These special cells are created by stem cells that hide deep in pockets of the intestine called crypts. When the right trigger signal comes along, such as a worm infection, the production of tuft cells soars.
However, the new study shows that bacteria that normally live in the intestine can play an inhibiting role that prevents stem cells from making tuft cells in both mouse and human intestine. Certain microbes chewing on dietary fiber emit butyrate, a common form of short-chain fatty acid that is often sold in health food stores as a potentially helpful dietary supplement.
“Gut bacteria that emit lots of butyrate inhibit the activity of a protein called HDAC3, which stem cells need for making tuft cells,” Eshleman says. “So, too much butyrate can mean not enough tuft cells—and that can be bad news when a helminth infection comes along.”
Next steps
Much more research is needed before treatments aimed at controlling tuft cell numbers or function could be ready for patients, Alenghat says. One important future line of study will be seeking more understanding of how the life-long interplay of factors that change the microbiome also “train” the immune system, in good ways and bad.
“The microbiome colonization process begins at birth, which suggests that there’s a very early window that can set the course for microbiome-cell interactions for years to come,” Alenghat says.
Looking ahead, the paper suggests three possible ways to influence tuft cell production and potentially control the inflammation that occurs during chronic inflammatory conditions.
- Dietary regimens that can control fiber intake, up or down, can influence HDAC3 activity and change the number of tuft cells.
- Microbe-produced metabolites might be used to calibrate stem cell responses in the gut.
- Also, introducing new colonies of gut bacteria might be useful if people lack enough of the right types of bacteria.
Successful treatment regimens down the road would likely require highly personalized approaches to re-calibrate, or re-balance the dynamics within the microbiome in healthy ways.
“It’s basically a constant balancing act,” Alenghat says. “One of the struggles our bodies have is balancing the protective vs. pathologic aspects of our immune systems.”
About the study
In addition to Eshleman and Alenghat, Cincinnati Children’s co-authors included: Taylor Rice, Crystal Potter, Amanda Waddell, PhD, Seika Hashimoto-Hill, DVM, PhD, Vivienne Woo, PhD, Sydney Field, Laura Engleman, and Fred Finkelman, MD, all with the Division of Immunobiology; Hee-Woong Lim, PhD, Division of Biomedical Informatics; and Lee Denson, MD, Division of Gastroenterology, Hepatology, and Nutrition.
Funding sources for this research include several grants to investigators from the National Institutes of Health (DK114123, DK116868, DK095004, DK119694, K01DK131390, F32AI147591 and K01DK135647), the Kenneth Rainin Foundation, and the Burroughs Wellcome Fund. This project also is supported by the Center for Stem Cell & Organoid Medicine at Cincinnati Children’s and NIH grants to the Digestive Health Center (P30 DK078392) and the Molecular Hematology Center of Excellence (U54 DK126108).
Watch a TedX Cincinnati presentation from Dr. Alenghat:

| Original title: | Microbiota-derived butyrate restricts tuft cell differentiation via histone deacetylase 3 to modulate intestinal type 2 immunity |
| Published in: | Immunity |
| Publish date: | Jan. 30, 2024 |
Research By


The Alenghat Lab investigates epithelial and immune cell homeostasis in the context of intestinal health and disease.