Breaking Down the Build-Up: Enhancing Brain GCase in Gaucher Disease
Research By: Ying Sun, PhD | Xiaoyang Qi, PhD
Post Date: October 5, 2021 | Publish Date: April 10, 2020


Experts recap a multi-pronged hunt to improve treatment for a rare neurologic disorder
Many patients with the inherited metabolic disorder Gaucher Disease do not have enough properly working glucocerebrosidase (GCase), the digestive enzyme that is responsible for the breakdown of the lipid glucocerebroside.
The absence of fully functioning GCase leads to a toxic accumulation of these lipids, especially in the brain, peripheral nervous system, liver, spleen, and bone marrow. Neuronopathic (Type 2 and Type 3) Gaucher disease are characterized by problems that affect the brain and spinal cord, which cause life-threatening medical problems. The neurological complications of lipid build-up include lack of muscle coordination, feeding and swallowing difficulties, pain, loss of muscle tone, seizures and brain degeneration.
Ying Sun, PhD, Division of Human Genetics at Cincinnati Children’s, and her collaborators are targeting multiple therapeutic methods to address the need for treatment of patients with neuronopathic Gaucher disease.
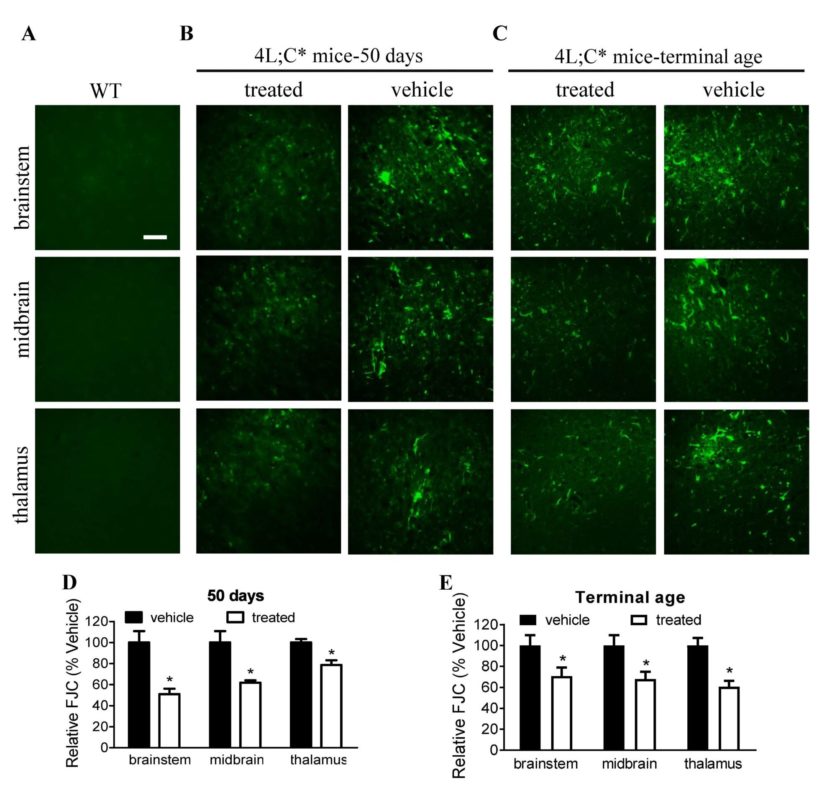
Enzyme replacement therapy
GCase enzyme replacement therapy (ERT) can have positive effects against lipid build-up in the visceral organs; however, these replacement enzymes do not cross the blood brain barrier to reach the central nervous system (CNS). Aware of this challenge, Sun found a collaborator Xiaoyang Qi, PhD, Professor of Medicine and Co-Division Chief for Basic Science Research at the University of Cincinnati Cancer Institute.
In 2002, while studying Gaucher disease at Cincinnati Children’s, Qi discovered a combination of a lysosomal protein, saposin C (SapC), and a phospholipid that, assembled into tiny cavities, or nanovesicles, can kill many forms of cancer while leaving healthy cells unaffected*. Moreover, this nanovesicle can deliver drugs/agents for cancer therapy and imaging.
In Sun and Qi’s experiments, nanovesicles composed of SapC and dioleoylphosphatidylserine (DOPS) were used to deliver functional GCase to CNS cells in a Gaucher disease mouse model. This non-invasive, localized method of ERT enabled GCase’s function of breaking down lipids in the CNS.
When integrated into these nanovesicles, functional GCase enzymes are more efficiently delivered to CNS cells, resulting in higher GCase activity and reduced GCase substrate levels where needed most. Increased GCase activity above untreated affected control levels produced profound improvement in brain inflammation and neurological symptoms.
Stem Cell Therapy
Another approach to increase GCase activity employs intravenous delivery of neural precursor cells to develop into a variety of helpful cell types that provide normal enzyme to the brain. Studies in mice receiving the induced pluripotent stem cell (iPSC)-derived neural precursor cells have shown increased GCase activity and decreased substrate levels in the brain. The use of iPSC-derived neural precursor cells also enhanced expression of neurotrophic factors that have roles in neuronal survival, the promotion of neurogenesis and in improving sensorimotor function, which support the feasibility of autologous neural precursor cell therapy.
Small molecule approach
In addition to ERT and stem cell solutions, small molecule non-inhibitory chaperone drugs are being developed to improve the function of existing GCase in the body. A pharmacological chaperone is a drug that acts as a protein “scaffold” to stabilize misfolded mutant proteins and direct them to act correctly within the cell. The majority of pharmacological chaperones that have been previously investigated are inhibitory in nature and focused on preventing premature degradation of GCase.
This mechanism of action has been challenging as it often has a negative effect on enzyme activity. Non-inhibitory chaperones work to restore normal function of existing GCase in the body and improve activity. Current studies have shown these non-inhibitory chaperones are capable of increasing GCase activity to a therapeutic level close to the activity in healthy people. Their increased ability to penetrate the blood brain barrier makes them promising candidates for novel therapeutics for neuronopathic Gaucher disease.
Next steps
Given that there are currently no treatments for neuronopathic Gaucher disease, this research shows considerable promise of therapeutic benefit. Dr. Sun and Dr. Qi’s goal is to further develop effective treatments for neuronopathic Gaucher disease and other related neurologic conditions, e.g. Gaucher disease-associated Parkinson disease.
Future activities will include optimizing those agents to ensure they are safe and effective, ultimately enabling the candidates for future clinical studies in neuronopathic Gaucher disease and related disease conditions.
See related research: Intravenous infusion of iPSC-derived neural precursor cells increases acid β-glucosidase function in the brain and lessens the neuronopathic phenotype in a mouse model of Gaucher disease, Human Molecular Genetics, October 2019.
For more information about the status of these technologies, please visit Innovation.CincinnatiChildrens.org.
*SapC-DOPS was licensed by Cincinnati Children’s to Bexion Pharmaceuticals in 2006.
| Original title: | Systemic enzyme delivery by blood-brain barrier-penetrating SapC-DOPS nanovesicles for treatment of neuronopathic Gaucher disease |
| Published in: | EBioMedicine |
| Publish date: | April 10, 2020 |






