Borrowing Historical Controls Could Accelerate Rare Disease Research
Research By: Nusrat Harun, PhD | Maurizio Macaluso, MD, DPH
Post Date: March 17, 2023 | Publish Date: Mar. 17, 2023
Biostatistics and Epidemiology | Top Scientific Achievement

Rare disease research faces challenges due to limited patient populations, making randomized controlled trials slow and costly. However, clinical trials could be accelerated through the use of historical controls alongside concurrent controls, according to researchers from Cincinnati Children’s.
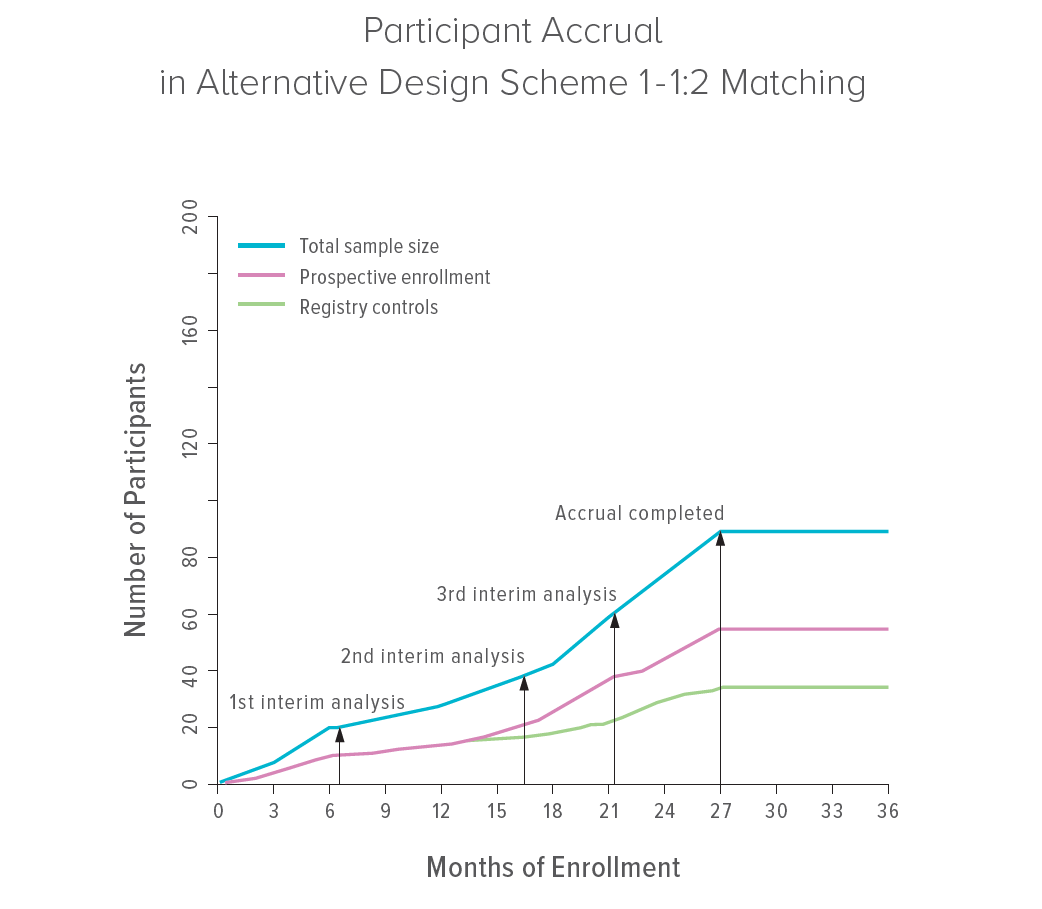
The study, led by first author Nusrat Harun, PhD, and senior author Maurizio Macaluso, MD, DrPH, re-analyzed data from the MILES trial led by Francis McCormack, MD, at the University of Cincinnati, which resulted in the FDA’s approval of sirolimus for treating lymphangioleiomyomatosis (LAM). They used propensity score matching to identify comparable historical controls from a registry, allowing for more new patients to be assigned to the sirolimus arm. Statistical simulations indicated that borrowing information from historical controls would have led to the same trial conclusions with fewer concurrent controls, without compromising statistical performance.
The MILES trial needed 964 days to enroll 89 patients and conduct the final analysis. In an efficient alternative design, however, the trial would have needed 34 fewer participants and could have been completed five months sooner in part by replacing a placebo arm with matched historical controls. The comparison between active treatment (sirolimus) and control would have yielded almost identical results as observed in the actual trial, leading to the same conclusion earlier and with lower costs.
“Our findings suggest that augmenting the design of a trial by incorporating high-quality historical control data (from previous clinical trials or natural history studies) can reduce the number of new patients needed in the control arm and accelerate patient enrollment,” Macaluso says. “It requires case-by-case consideration to assure the quality and rigor of the historical data and other factors, but this innovative approach could significantly benefit the field of rare disease research and expedite the development and evaluation of new treatments for these conditions.”
More 2023 Research Highlights
Chosen by the Division of Biostatistics and Epidemiology
Nidey N, Hoyt-Austin A, Chen MJ, et al. Racial Inequities in Breastfeeding Counseling Among Pregnant People Who Use Cannabis. Obstet Gynecol. 2022;140(5):878-881. doi:10.1097/AOG.0000000000004834
Steen DL, Helsley RN, Bhatt DL, et al. Efficacy of supermarket and web-based interventions for improving dietary quality: a randomized, controlled trial. Nat Med. 2022;28(12):2530-2536. doi:10.1038/s41591-022-02077-7
Zhou GC, Song S, Szczesniak RD. Multilevel joint model of longitudinal continuous and binary outcomes for hierarchically structured data. Stat Med. 2023;42(17):2914-2927. doi:10.1002/sim.9758
Gecili E, Brokamp C, Rasnick E, et al. Built environment factors predictive of early rapid lung function decline in cystic fibrosis. Pediatr Pulmonol. 2023;58(5):1501-1513. doi:10.1002/ppul.26352
Szczesniak R, Andrinopoulou ER, Su W, et al. Lung Function Decline in Cystic Fibrosis: Impact of Data Availability and Modeling Strategies on Clinical Interpretations. Ann Am Thorac Soc. 2023;20(7):958-968. doi:10.1513/AnnalsATS.202209-829OC
View more discoveries from 50 research divisions and areas
Return to the 2023 Research Annual Report main features
| Original title: | Dynamic use of historical controls in clinical trials for rare disease research: A re-evaluation of the MILES trial |
| Published in: | Clinical Trials |
| Publish date: | Mar. 17, 2023 |
Research By