A Quest to Unlock the Secrets of Eosinophilic Disorders
Research By: Marc Rothenberg, MD
Post Date: June 27, 2019 | Publish Date: January 2015
Top Research Achievements 2010-2015
Since 2010, scientists at Cincinnati Children’s have produced more than 7,000 peer-reviewed studies with far-reaching impact across pediatric medicine. From this impressive body of work, six advances stand out as Cincinnati Children’s premier scientific achievements of 2010-15.

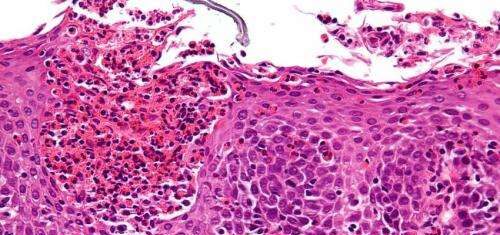
The mission started because scientists wanted to find out why some children develop food allergies so severe that it affects their growth. These children suffer frequent stomachaches vomiting and diarrhea. They have trouble swallowing and often must endure life on strictly limited diets. The search for answers has changed our understanding of food allergies and established a newly recognized set of conditions, called eosinophilic gastrointestinal disorders (EGID), in which the immune system treats food as a foreign invader.
Until the last decade, eosinophils were best known as end-stage cells of the immune system. Their only acknowledged job was to provide host protection against parasites. However, Marc Rothenberg, MD, PhD and colleagues have shown that eosinophils are multifunctional leukocytes involved in diverse inflammatory responses. The work progressed from describing a single condition — eosinophilic esophagitis (EoE) — to establishing an entire class of disorders. Rothenberg coined the term EGID in 2004, coinciding with him becoming the Founding Director of the Cincinnati Center for Eosinophilic Disorders, the first center of its kind in the U.S. Rothenberg has published over 300 papers researching these topics, including many that have deeply influenced the field.
Rothenberg’s team has characterized several critical pathways that regulate allergic responses, including the eotaxin family of chemokines (eosinophil chemoattractants) and interleukin 5 (eosinophil growth/activating cytokine), which is now a proven target for eosinophilic subtypes of asthma. His laboratory established the first animal models of EoE and identified the EoE transcriptome, a genome-wide set of molecular markers that have been translated into a commercially available EoE diagnostic panel. Rothenberg’s team conducted the first controlled clinical trial in EoE, demonstrating the effectiveness of topical glucocorticoid therapy, which is now in clinical use.
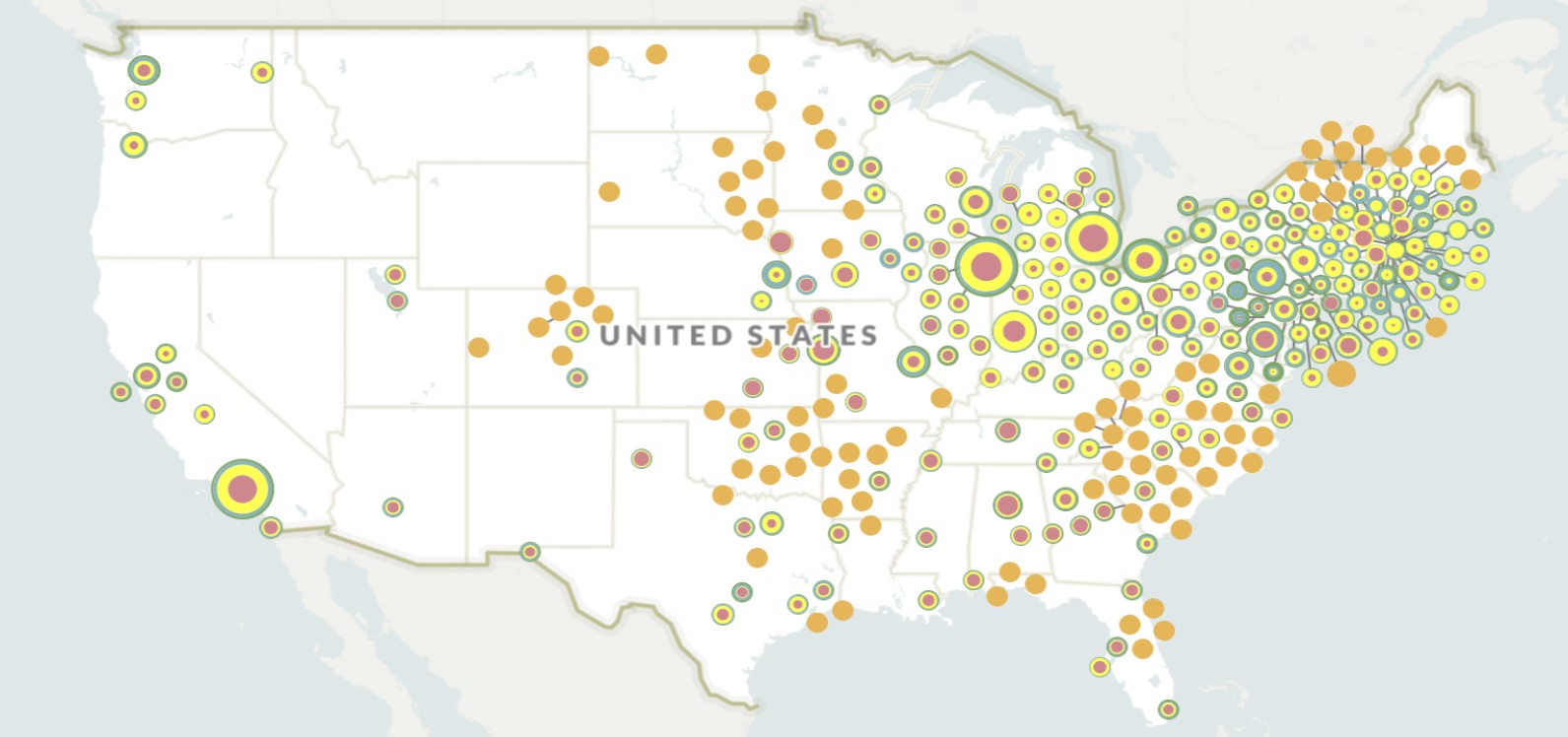
In 2010, Rothenberg published the first genome-wide association study of EoE in Nature Genetics. This study demonstrated a genetic linkage with chromosome 5q22 and that genetic variants of the immune hormone thymic stromal lymphopoietin were involved in allergen sensitization.
With John Harley, MD, PhD, Leah Kottyan, PhD, and other collaborators at Cincinnati Children’s, Rothenberg conducted another genome-wide analysis that interrogated more than 1.5 million genetic variants in EoE. The team published a breakthrough in 2014 in Nature Genetics identifying a powerful association between EoE and chromosome region 2p23, particularly in the gene CAPN14.
They found that CAPN14 was located in an epigenetic “hotspot” modified by IL-13 and was dynamically upregulated as a function of disease activity. Remarkably, the CAPN14-encoded protein, calpain 14, was specifically and most highly expressed in the esophagus, answering the long-standing question in the allergy field of why patients develop tissue-specific manifestations.
Visit the Rothenberg lab
(This story originally appeared in the 2015 Research Annual Report)
Research By