Gene Therapy Advances Raise Hopes for Curing Sickle Cell Disease
Research By: Punam Malik, MD
Post Date: June 27, 2019 | Publish Date: January 2015

Top Research Achievements 2010-2015
Since 2010, scientists at Cincinnati Children’s have produced more than 7,000 peer-reviewed studies with far-reaching impact across pediatric medicine. From this impressive body of work, six advances stand out as Cincinnati Children’s premier scientific achievements of 2010-15.
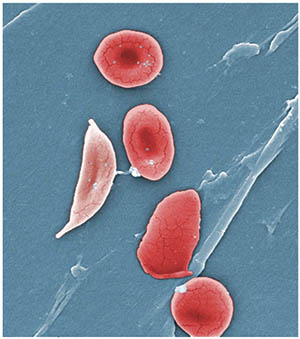
For many years, clinicians could do little more than try to manage the pain of sickle cell disease and treat complications as they occurred. For patients, it has been a world of daily antibiotics as young children, blood transfusions, hospital admissions, and — for those lucky enough to find a matching donor — bone marrow transplants. Now, scientists are closing in on a potential cure. Human clinical trials have begun to evaluate a patented treatment that could reshape the future for children born with sickle cell disease and represent a major step forward for the field of gene therapy.
Developed at Cincinnati Children’s by Punam Malik, MD, the treatment employs a modified lentivirus to transfer a healthy fetal hemoglobin gene to people with sickle cell disease, which can allow their bodies to produce normal red blood cells instead of the sickle-shaped cells that define the inherited disorder. If successful, the treatment would effectively cure a disease that affects more than 90,000 people in the United States and millions of people worldwide. It also may serve as a treatment for thalassemia, a related rare blood disease.
A U.S. patent application was filed in 2011 for the gene therapy method after years of research led by Malik demonstrated that the technique was effective in halting blood cell sickling in mice and in human tissue samples.
In sickle cell disease, a genetic defect makes red blood cells carry hemoglobin S, which produces misshapen blood cells that cannot efficiently deliver oxygen. Over time, the condition can trigger strokes, kidney failure and other forms of tissue damage.
Malik’s innovation flows from the observation that some adults never stop producing fetal hemoglobin (HbF), which prevents red blood cell sickling when present in the body in sufficient quantity. Normally, the fetal hemoglobin gene switches off shortly after birth. The new gene therapy reactivates it.
The clinical trial, which envisions following 10 participants for 15 years, began enrolling participants in 2014. The treatment involves collecting bone marrow stem cells from patients, using a lentivirus vector to deliver a healthy γ-globin gene to the cells, then returning the engineered cells to the patients where they can multiply into permanent HbF factories.
The technique patented at Cincinnati Children’s stands out because it restricts transgene expression to maturing erythroid cells, which reduces the cancer risks that have plagued other gene therapy efforts.
It remains unclear whether gene therapy will become the ultimate cure for sickle cell disease. Newer technologies, including the CRISPR/Cas9 gene editing system, may be able to directly correct the sickle cell genetic defect in hematopoietic stem cells without the need for gene insertion therapy.
Learn more about Malik’s sickle cell research
(This story originally appeared in the 2015 Research Annual Report)
Research By







