Blood Cell Production Discovery Opens Path to Improve Anemia of Inflammation
Research By: Laurel Romano, PhD | Katie Seu, PhD | Theodosia Kalfa, MD, PhD
Post Date: October 31, 2022 | Publish Date: Oct. 6, 2022
Journal cover features study led by experts at Cincinnati Children’s explaining the inner workings of newly termed ‘erythromyeloblastic islands’ (EMBI)
Since 1958, scientists have known that red blood cell production in the bone marrow involves clusters of several developing red blood cells that surround a larger white blood cell called a macrophage.
These clusters have been called erythroblastic islands (EBI) and they are the first discovered hematopoietic niches. But now they should be recognized as erythromyeloblastic islands (EMBI), niches for terminal erythropoiesis and myelopoiesis, according to a discovery published in the latest issue of Blood.
The study, which included 16 co-authors from four institutions was led by co-first authors Laurel Romano, PhD, Division of Hematology, and Katie Seu, PhD, Division of Experimental Hematology and Biology at Cincinnati Children’s, and senior author Theodosia Kalfa, MD, PhD, Division of Hematology and co-director of the Erythrocyte Diagnostic Laboratory at Cincinnati Children’s.
“This discovery shifts the paradigm of what erythroblastic islands are, revealing that not only erythroblasts, but also granulocytes, utilize this niche for maturation,” Kalfa says.
The study reports that the previously unknown presence of granulocyte precursor cells within EBIs changes the map of these blood cell islands. Now, when these islands are disrupted by gene variations or external stresses, it appears possible for the red versus white blood cell production balance within EMBIs to be shifted.
Implications for anemia of inflammation
For example, overproducing granulocytes can prevent production of red blood cells, leading to a condition called “anemia of inflammation.” This form of anemia occurs in millions of people worldwide as an outcome of multiple health conditions including cancer, rheumatoid arthritis, severe infections and sepsis, and chronic or acute immune activation.
So far, there have been no successful targeted treatments.
“Treatment for anemia of inflammation relies on blood transfusions or resolution of the underlying disease, which often is not feasible,” Kalfa says. “Our findings offer insights of how the anemia occurs. Understanding the mechanism of a disease is a basic step to eventually discover novel treatment targets.”
Discovery required better tools for studying cells
One reason why other researchers did not discover the M in EMBI sooner is that the cell-to-cell interactions within these islands are so tight that previous methods were unable to visualize clearly these structures or isolate their component cells.
“We developed a method to enrich for erythroblastic islands and then used cutting-edge techniques to image them as intact clusters in a high-throughput manner using imaging flow cytometry,” Kalfa says.
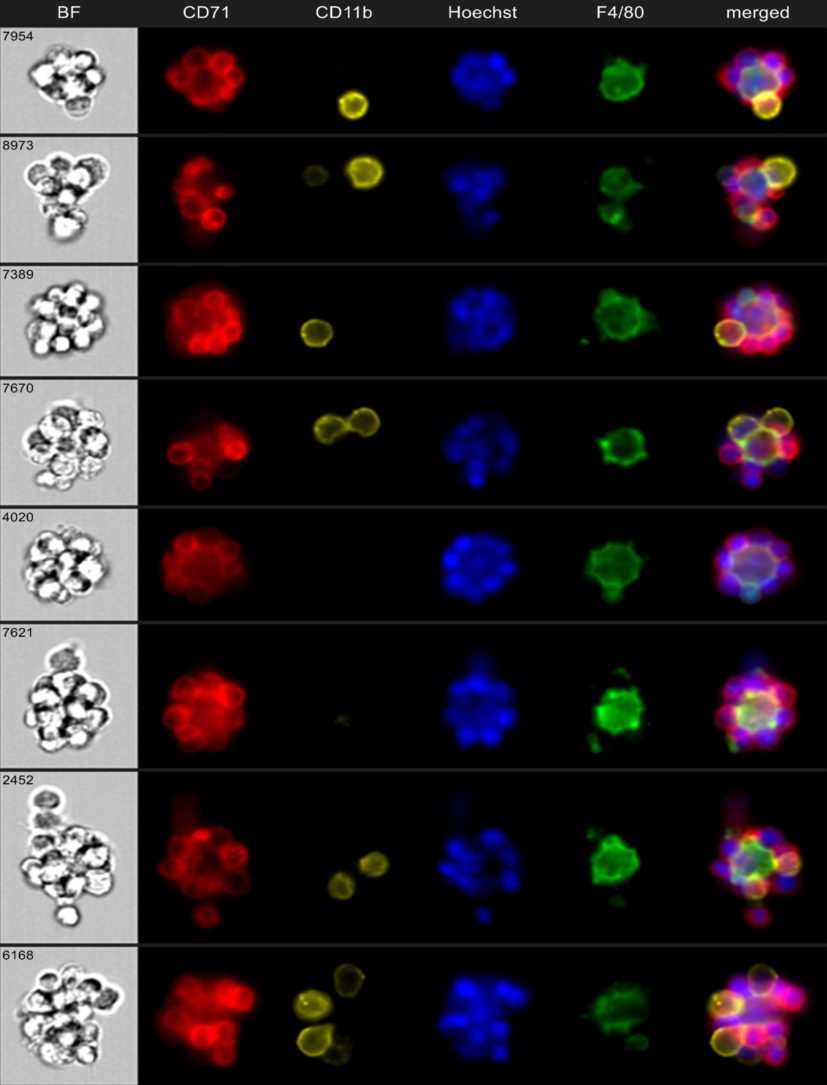
Using mouse models that mimic the conditions that cause anemia of inflammation in humans, such as sepsis, inflammatory bowel disease (IBD), and arthritis, the team revealed that the erythroblastic island macrophages act as a fulcrum in the production balance between red blood cells, which carry oxygen to the tissues, and white blood cells, which fight infection. Researchers were able to use granulopoiesis and erythropoiesis stimulating agents to shift the fulcrum towards either red or white blood cell maturation.
Next steps
Future studies will seek to understand the specific mechanisms and signals that disease states can trigger to unbalance this newly documented system. From that work, existing drugs or new ones may be able to prevent or treat anemia of inflammation, which worsens the quality of life in millions of patients with chronic disease.
Meanwhile, the elaborate single-cell RNA sequencing studies and imaging flow cytometry processes developed to study blood cell production may be useful to other researchers seeking to unravel tightly tangled cell function mysteries.
About the study
Co-authors from Cincinnati Children’s included: Yi Zheng, PhD, H. Leighton Grimes, PhD, David Muench, PhD, Diamantis Konstantinidis, PhD, Andre´ Olsson, PhD, Kashish Chetal, MSc, and Katrina Schlum, PhD.
Collaborators also included Lionel Blanc, PhD, co-senior author in this study, Julien Papoin, MSc, and Betsy Barnes, PhD, from The Feinstein Institutes for Medical Research, Manhasset, NY; Joel Anne Chasis, MD, from Lawrence Berkeley National Laboratory; and Narla Mohandas, DSc, from the New York Blood Center.
Funding sources for this study include grants from the National Institutes of Health (R01HL152099, R01HL122661, and U54 DK126108)
| Original title: | Erythroblastic islands foster granulopoiesis in parallel to terminal erythropoiesis |
| Published in: | Blood |
| Publish date: | Oct. 6, 2022 |
Research By