Where Rare Isn’t So Rare: Clinical Sites Provide Specialized Care for Rare Disease Patients
Post Date: February 27, 2022 | Publish Date:

When a child is diagnosed with a rare disease, caregivers often search for a place where they can receive specialized care. At these places, rare diseases aren’t so rare—they are examined and studied every day.
For many patients and families, that place is Cincinnati Children’s. We host several clinical sites within the National Institutes of Health-funded Rare Diseases Clinical Research Network (RDCRN), a national network of physicians, researchers, patients, and advocates working together to advance medical research on rare diseases.
From all over the world, patients and families travel to our clinics for cutting-edge care. Here, clinicians are not only familiar with rare diseases—they are experts paving the way for better treatments, outcomes, and cures.
Watch at WKRC: A local mom’s mission after her daughter’s rare disease diagnosis.
Read about how the Cincinnati Zoo honors Oliver, a boy who battled VACTERL association
Currently, Cincinnati Children’s hosts four RDCRN clinical sites—specializing in brain vascular malformations, eosinophilic gastrointestinal disorders, developmental synaptopathies, and primary immune deficiencies—as well as the network’s Data Management and Coordinating Center, which facilitates network operations and research.
Brain Vascular Malformation Consortium (BVMC)
“We see pediatric as well as adult patients, and we are a destination center for providers outside of Cincinnati Children’s. Our involvement in the RDCRN allows expertise on the disease topic and leadership in clinical research participation.”
—Sudhakar Vadivelu, DO
Our Experts:
- Adrienne Hammill, MD, PhD; research director, Hemangioma & Vascular Malformation Program; director, HHT Center of Excellence; director, Sturge-Weber Center/Clinical Care Network
- Sudhakar Vadivelu, DO; director, Cerebrovascular Disease and Stroke Center
Diseases We Study:
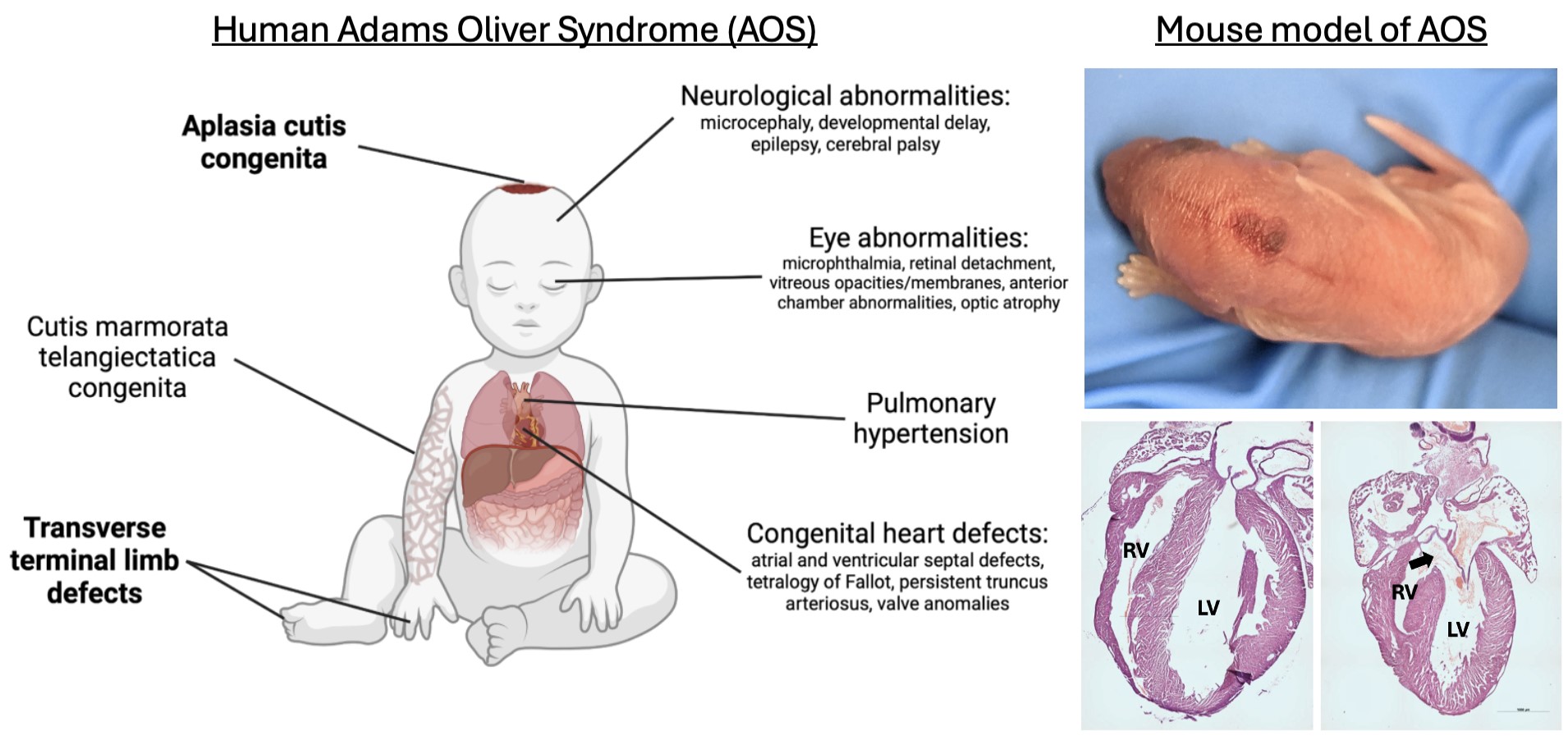
- Cerebral cavernous malformations (CCM), abnormally enlarged spaces in the brain where blood collects near irregularly shaped, enlarged capillaries (tiny blood vessels) which have abnormally thin walls prone to leaking; symptoms include headaches, seizures, paralysis, hearing or vision loss, and cerebral hemorrhaging (brain bleeds)
- Hereditary hemorrhagic telangiectasia (HHT), a disorder categorized into 3 types in which blood flows from high pressured arteries directly into low pressured veins (arteriovenous malformations) instead of going through capillaries which act as a pressure buffer; symptoms include tissue irritation, frequent nosebleeds, hemorrhaging, and visible red markings (telangiectasias)
- Sturge-Weber syndrome (SWS), a condition resulting in abnormal blood vessel development in the brain, eyes, and skin at birth; symptoms include migraines, seizures, vision problems, and hemiparesis (temporary one-sided muscle weakness)
Current Studies:
- Modifiers of Disease Severity and Progression in Cerebral Cavernous Malformation: Collecting clinical, genetic, imaging, and environmental information for people with familial CCM1 to look for risk factors affecting CCM disease severity and progression
- Integrated Longitudinal Studies to Identify Biomarkers and Therapeutic Strategies for Sturge-Weber Syndrome: Integrating clinical data, radiological data, and blood biomarkers of patients to understand more about Sturge-Weber Syndrome, the possible treatments for this disease, and identify targets for clinical trials
- Cerebral Hemorrhage Risk in Hereditary Hemorrhagic Telangiectasia: Studying risk factors for rupture of brain arteriovenous malformations (BAVMs), bleeding, and other features of HHT, including primarily genetics and imaging characteristics of the BAVMs
Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR)
“CEGIR provides unparalleled access to a large database of pathology data of a rare disease. We have participated in studies with meaningful results that would not have been possible without CEGIR.”
—Margaret Collins, MD
Our Experts:
- Marc Rothenberg, MD, PhD; director, Cincinnati Center for Eosinophilic Disorders
- J. Pablo Abonia, MD; associate professor, Division of Allergy and Immunology, Eosinophilic Disorders
- Scott Bolton, MD; attending physician, Division of Gastroenterology, Hepatology and Nutrition
- Margaret Collins, MD; staff pathologist, Division of Pathology; director, GI Pathology
- Lisa Martin, PhD; professor, Human Genetics
- Vincent Mukkada, MD; medical director, Lumen Inpatient Unit, A4S
- Philip Putnam, MD; director, Endoscopy Services
Diseases We Study:
- Eosinophilic disorders, which occur when eosinophils (white blood cells of the immune system) are found in above-normal amounts in various parts of the body, causing chronic inflammation resulting in tissue damage; diagnosed according to the location where the levels of eosinophils are elevated:
- Eosinophilic esophagitis (esophagus)
- Eosinophilic gastritis (stomach)
- Eosinophilic gastroenteritis (stomach and small intestine)
- Eosinophilic enteritis (small intestine)
- Eosinophilic colitis (large intestine)
- Hypereosinophilic syndrome (blood and any organ)
Current Studies:
- OMEGA-A Prospective, Multicenter Study to Compare and Validate Endoscopic, Histologic, Molecular and Patient-Reported Outcomes in Pediatric and Adult Patients with Eosinophilic Esophagitis (EoE), Gastritis (EG), Gastroenteritis (EGE) and Colitis (EC): Comparing patients’ symptoms with their tissue samples
- Use of Unsedated Transnasal Esophagoscopy to Monitor Dietary Management of Eosinophilic Esophagitis in Children: Using unsedated transnasal endoscopy (TNE) to learn more about how long it takes esophageal eosinophils to come back after either a new food is started or swallowed topical steroid therapy is removed in children with eosinophilic esophagitis (EoE)
- A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Evaluate the Efficacy of Dupilumab (ANTI-IL4RA) in Subjects with Eosinophilic Gastritis: Determining if dupilumab helps improve inflammation in the stomach of people with eosinophilic gastritis (EG)
- Pilot Assessment to Find Evidence of Gastric Motility Abnormalities in Eosinophilic Gastric Disorders (OAT-FEED Study): Comparing the relationship between stomach emptying (the time it takes for food to leave the stomach) with stomach tissue samples
Developmental Synaptopathies Consortium (DSC)
“Pediatric neurology continues to be a medical specialty where discoveries and new treatments are exploding. This is particularly true for many rare genetic diseases, which I focus on in my clinical practice and research.”
—Darcy Krueger, MD, PhD
Our Expert:
- Darcy Krueger, MD, PhD; director, Tuberous Sclerosis Clinic
Diseases We Study:
- Tuberous sclerosis complex, a genetic condition in which typically benign tumors affect multiple organs including the brain, kidneys, heart, lungs, eyes, and skin; symptoms include skin abnormalities, seizures, developmental delay, intellectual disability, and renal failure
- Phelan-McDermid syndrome, a disorder caused by loss of a portion of chromosome 22 near its end at a location designated q13.3; symptoms include hypotonia (low muscle tone), global developmental delay, intellectual disability, motor skills delay, delayed or absent speech, mild facial dysmorphism (abnormal structure), seizures, and autism spectrum disorder (ASD)
- PTEN hamartoma tumor syndrome, a spectrum of disorders characterized by the widespread presence of multiple hamartomas (localized, benign, tumor-like malformations) which contain a mixture of mature cells and tissue; affected individuals may have an increased risk for certain cancers and neurodevelopmental disorders
Current Studies:
- Autism Spectrum Disorder (ASD) and Intellectual Disability (ID) Determinants in Tuberous Sclerosis Complex (TSC): Exploring earlier signs of ASD and ID in patients with TSC to gain a better understanding for effective treatments and interventions
- Natural History Study of Individuals with Autism and Germline Heterozygous PTEN Mutations: Learning more about the symptoms and characteristics of people with ASD and/or PTEN mutations to gain information about risk management, as well as identify biomarkers for intervention studies
Primary Immune Deficiency Treatment Consortium (PIDTC)
“Our physicians not only provide state-of-the-art treatment for patients, but they also lead the field with primary immune deficiency research and new treatment trials.”
—Rebecca Marsh, MD
Our Experts:
- Jacob “Jack” Bleesing, MD, PhD; co-director, Diagnostic Immunology Laboratories; associate director, Immunodeficiency and Histiocytosis Program
- Rebecca Marsh, MD; clinical director, Primary Immune Deficiency Program; co-director, Diagnostic Immunology Laboratories; clinical director, HLH Center of Excellence
Diseases We Study:
- Primary immune deficiency, a term describing a group of over 400 rare, chronic disorders characterized by weakened or absent immune systems that cannot properly protect the body from viruses, fungi, or bacteria, including:
Current Studies:
- A Prospective Natural History Study of Diagnosis, Treatment, and Outcomes of Children with Severe Combined Immune Deficiency (SCID) Disorders: Studying new patients undergoing treatment for SCID to learn about long-term outcomes, impact of newborn screening, early diagnosis, and gene therapy
- A Retrospective and Cross-Sectional Analysis of Patients Treated for Severe Combined Immune Deficiency (1968-2010): Investigating long-term outcomes (such as infections, graft-versus-host disease, autoimmune diseases, and quality of life) in SCID patients who have undergone blood and marrow transplant, gene therapy, or enzyme replacement
- Analysis of Patients Treated for Chronic Granulomatous Disease (CGD) Since January 1, 1995: Exploring the role of blood and marrow transplant compared to medical management of CGD
- Analysis of Patients Treated for Wiskott-Aldrich Syndrome (WAS) Since January 1, 1990: Investigating outcomes for patients with WAS who have or will be receiving either a blood and marrow transplant or gene therapy
Read about the RDCRN Data Management and Coordinating Center at Cincinnati Children’s.
The Rare Diseases Clinical Research Network (RDCRN) is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Office of Rare Diseases Research (ORDR). Now in its fourth five-year funding cycle, RDCRN is a partnership with funding and programmatic support provided by Institutes, Centers, and Offices across NIH, including the National Institute of Neurological Disorders and Stroke, the National Institute of Allergy and Infectious Diseases, the National Institute of Diabetes and Digestive and Kidney Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Heart, Lung, and Blood Institute, the National Institute of Dental and Craniofacial Research, the National Institute of Mental Health, and the Office of Dietary Supplements.