Vaccine Trials Expand, New Findings Shed Light on ACE2 Role
Post Date: November 12, 2020 | Publish Date:
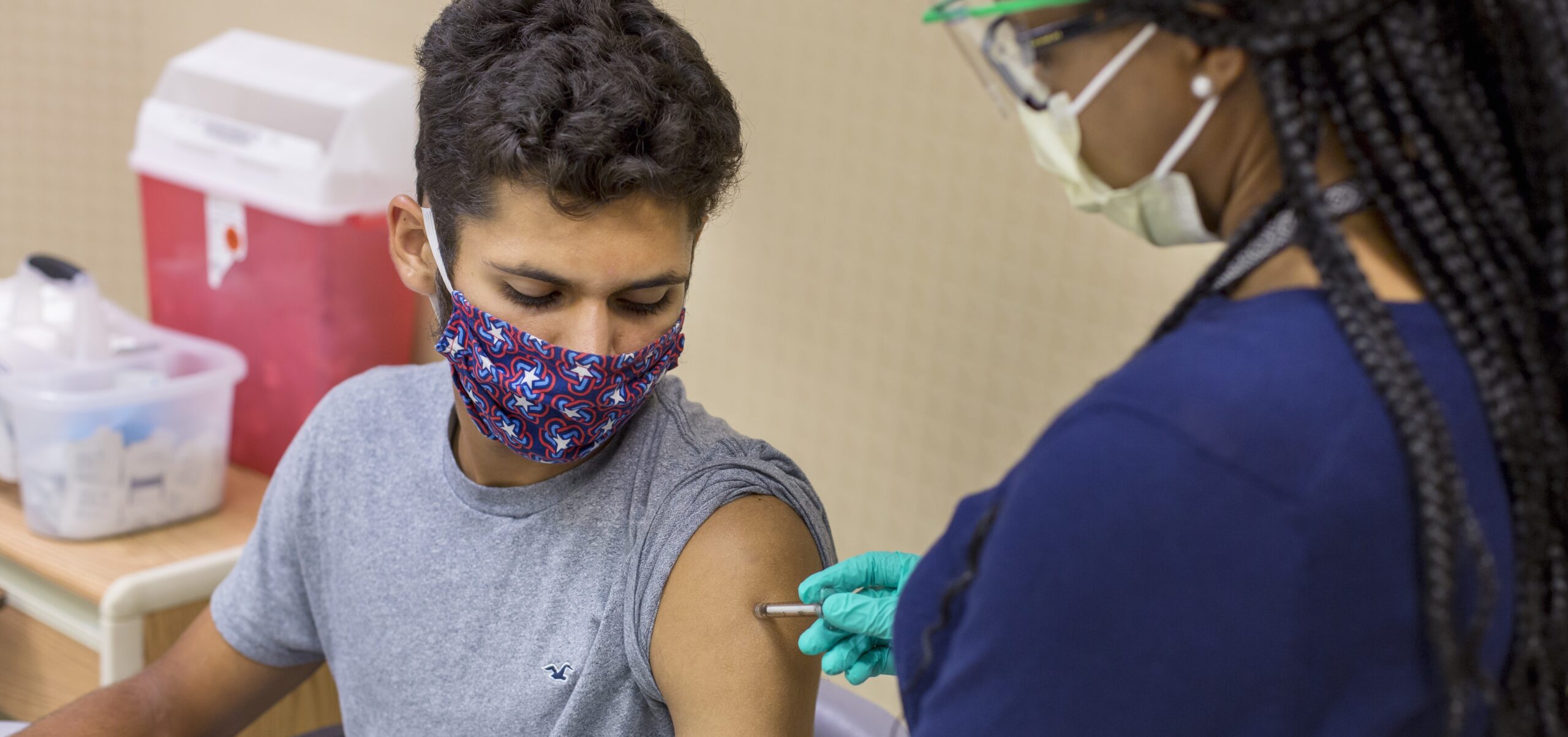
Signs of Progress for Pfizer-BioNTech Vax
Cincinnati Children’s has begun recruiting children ages 12 and up to participate in a clinical trial of a COVID-19 vaccine candidate that recently made headlines for demonstrating a new level of success.
The mRNA-based vaccine candidate, BNT162b2, made by Pfizer Inc. and BioNTech SE, was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection, according to a Nov. 8, 2020, announcement from the companies. This first interim efficacy analysis was based on 94 confirmed cases of COVID-19 among 43,538 trial participants, 38,955 of whom had received a second dose.
Cincinnati Children’s is one of four sites in the United States conducting the Phase 3 study. Adults have been participating since May. Two expansions in October have added children ages 12-17 to the study.
Recruitment Begins for AstraZenica-Oxford Vax
Cincinnati Children’s is recruiting about 500 people to participate in a Phase 3 clinical trial of the AZD1222 COVID-19 vaccine candidate being developed by AstraZeneca in collaboration with the University of Oxford.
Study leaders here say they are focusing especially on gaining participation from first responders, people 65 or older, Blacks and Hispanics to ensure inclusion of those at higher risk of contracting the disease or becoming seriously ill.
This viral vector-based vaccine candidate uses a weakened version of adenovirus containing the genetic material of SARS-CoV-2 spike protein to stimulate an immune response.
More Data Emerges About the Role ACE2 Plays in COVID-19
The drug infliximab, which is often used to manage inflammation among people with inflammatory bowel disease (IBD), may also help aid recovery for certain people infected by COVID-19, according to a new study published online Nov. 5, 2020, in the journal Gastroenterology.
Normally, angiotensin-converting enzyme 2 (ACE2) activates a hormone that helps regulate blood pressure. However, in the gastrointestinal tract, ACE2 levels influence inflammation that can aggravate IBD symptoms.
In COVID-19 infections, the SARS-CoV-2 virus binds to ACE2 and uses it to invade cells and spread the virus. Although noted early on for its affects on lung tissue, the novel coronavirus also can cause potentially severe gastrointestinal symptoms.
In the new study, co-authors report that treatment with infliximab normalized ACE2 levels in the GI tract among people with IBD, which suggests that managing ACE2 levels for COVID-19 patients could help control inflammation damage caused by the virus.
The study’s senior author was Cedars-Sinai IBD expert Dermot McGovern, MD, PhD. Lee Denson, MD, Director, Schubert-Martin Inflammatory Bowel Disease Center at Cincinnati Children’s co-authored the study.
“Our results may provide a link between ACE2 levels and demographic features such as increasing age and body mass index which have been associated with worse COVID-19 outcomes, and suppport novel anti-inflammatory therapies such as JAK1/2 inhibitors which are currently being tested in clinical trials,” Denson says.
Other collaborating institutions included the Broad Institute of MIT and Harvard, Emory University School of Medicine, Children’s Healthcare of Atlanta, the Cleveland Clinic; and Janssen Research and Development LLC.