Targeting the BCAP Protein Could Help Manage Inflammation in IBD and Infection Response
Research By: Chandrashekhar Pasare, DVM, PhD
Post Date: November 16, 2020 | Publish Date: Nov. 16, 2020

“This study provides fundamental understanding of how macrophage regulate their responses to pathogens to provide an effective yet controlled inflammatory response and avoid damage of the host tissue”
— Chandrashekhar Pasare, DVM, PhD
A new study published online Nov. 16, 2020 in PNAS, reports an important clue as to how the immune system resolves inflammation and promotes tissue repair following inflammatory damage.
When macrophages sense bacterial pathogens such as E.coli, they activate an inflammatory response to get rid of the pathogen. However, this response needs to be kept in check to avoid damaging host tissue. New research led by experts at Cincinnati Children’s reports that macrophages utilize a molecule called BCAP to transition into a reparative state following clearance of infection.
Macrophages are among the first cells to sense invading pathogens and subsequently initiate an inflammatory immune response aimed at destroying the microbe. Uncontrolled inflammation can be detrimental to the host leading to diseases such as inflammatory bowel disease (IBD).
How Macrophages Modulate Inflammation
This prompted a research team led by first author Ricardo Irizarry-Caro, BSc, and senior author Chandrashekhar Pasare, DVM, PhD, Division of Immunobiology, to investigate how macrophages modulate inflammation following effective clearance of the pathogen.
They hypothesized that the negative regulation of inflammation might be intrinsic to the original sensing of the pathogen. Macrophages utilize Toll-Like Receptors (TLRs) to sense various pathogens, and activation of these receptors leads to early inflammation. The authors found that downstream of TLR activation, BCAP simultaneously activates a parallel pathway called the PI3K-AKT pathway, which acts to limit these inflammatory signals.
Specifically, researchers found that BCAP downstream of TLR activation inhibited two key proteins, GSK3b and FOXO1, thereby putting a cap on the inflammation triggered by pathogen sensing. Yet this negative regulation of inflammatory effectors was not enough to promote macrophages to undergo the reparative transition.
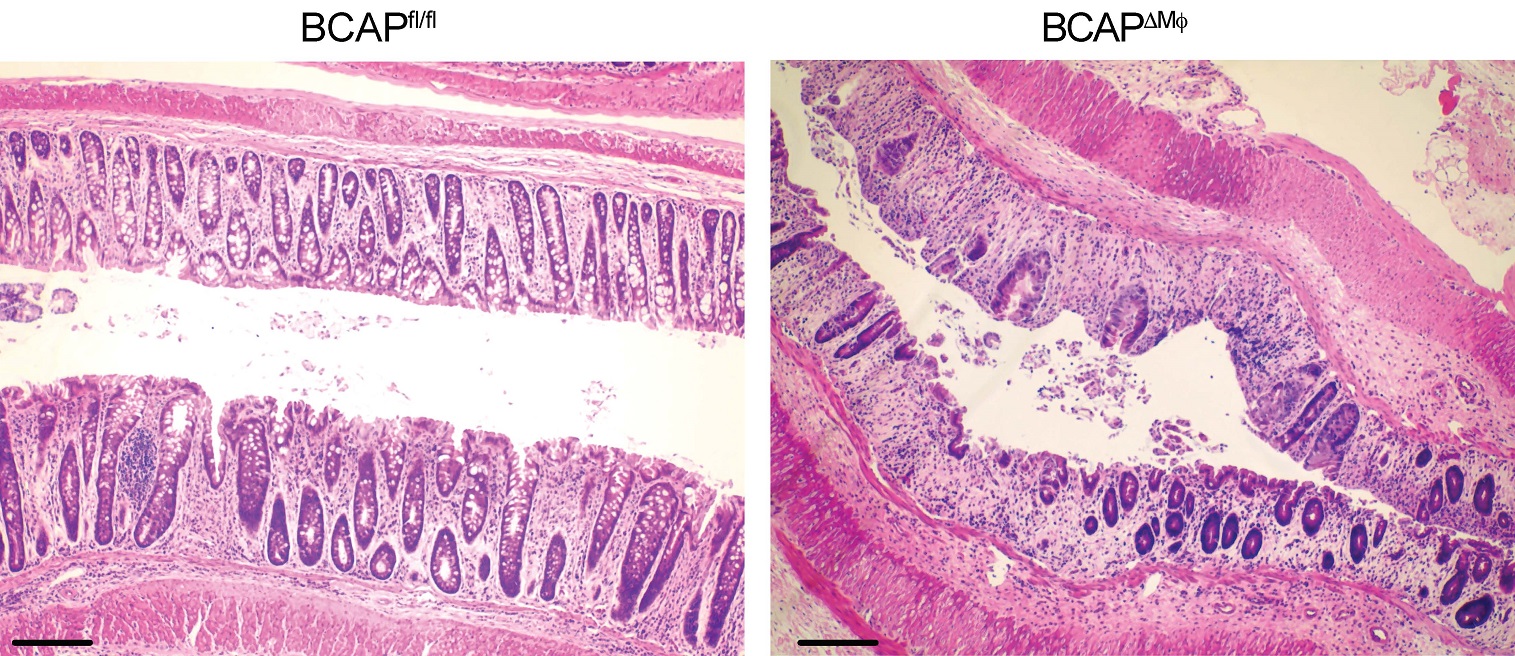
Researchers also found that BCAP was necessary to promote a metabolic process called glycolysis following TLR activation, which led to the production of lactate. This lactate is then incorporated into histones, proteins that are bound to DNA and regulated expression of certain genes including the ones that promote tissue repair. This process, called ‘histone lactylation’, promotes the reparative transition of macrophages and maintains tissue homeostasis following detection and clearance of a pathogen.
In this study, the authors demonstrate that intestinal repair after injury is critically dependent on BCAP expression in macrophages.
BCAP emerges as target for future drug research
“This study provides fundamental understanding of how macrophage regulate their responses to pathogens to provide an effective yet controlled inflammatory response and avoid damage of the host tissue” Pasare says. “More importantly, this study has implications for small molecules drug discovery targeting BCAP, which could be used to either promote reparative macrophage transition or induce a robust inflammatory response by just altering one single protein.”
This finding has implications for dampening inflammation in inflammatory diseases such as IBD and rheumatoid arthritis or promoting inflammation to drive immune responses against solid tumors.
About this study
Cincinnati Children’s conributors also include Margaret McDaniel, BSc, and Garrett Overcast, BSc, Division of Immunobiology. Other co-authors include Viral Jain, MD, Division of Neonatology, University of Alabama at Birmingham; and Ty Dale Troutman, PhD, Department of Cellular and Molecular Medicine, University of California at San Diego.
This work was supported by NIH Grants AI113125, AI123176, and GM120196.
| Original title: | TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation |
| Published in: | PNAS |
| Publish date: | Nov. 16, 2020 |
Research By