Study Challenges Current Thinking About Mechanisms of Dilated Cardiomyopathy
Research By: Kohta Ikegami, PhD
Post Date: June 11, 2024 | Publish Date: May 29, 2024
Research in Cell Reports led by experts at Cincinnati Children’s reveals nuclear envelope ruptures in heart muscle driving the disease
Scientists at Cincinnati Children’s report a significant breakthrough in understanding the mechanisms behind dilated cardiomyopathy (DCM), a common form of heart disease.
The study, published online May 29, 2024, in Cell Reports, focuses on LMNA-related DCM, a genetically inherited form of the disease. These cases are believed to account for 5% to 13% of idiopathic dilated cardiomyopathies (not linked to alcohol abuse, ischemia, infections, or other known factors).
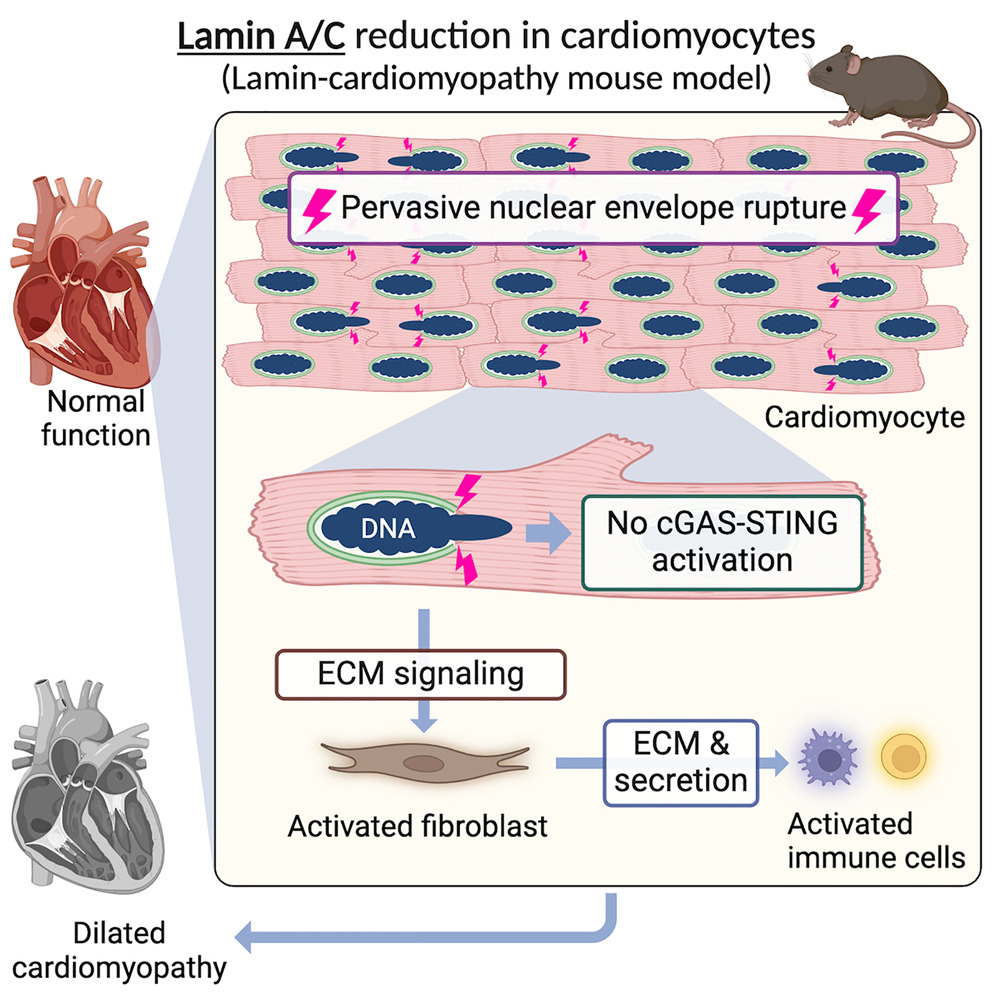
“This work is the first to establish that nuclear envelope rupture occurs frequently in the disease model heart before the heart starts becoming weak,” says corresponding author Kohta Ikegami, PhD, a member of the Division of Molecular Cardiovascular Biology at Cincinnati Children’s.
“Before this study, people had observed ruptures, but it was not known when and how often the ruptures happen, so it was unclear whether they could be the cause of the disease,” Ikegami says. “We found that almost 50% of heart muscle cells in the disease model heart have nuclear envelope ruptures compared to near zero found in normal hearts. This suggests that nuclear rupture is likely a disease cause.”
The study’s first author, Atsuki En, PhD, led the lab work that detected the high rates of nuclear envelope ruptures, Ikegami says.
Like finding a hidden tire puncture
The research team studied a line of mice that mimicked human disease by having the gene Lmna deleted specifically from heart muscle cells, known as cardiomyocytes. Within weeks, these mice developed heart failure.
In humans, DCM associated with malfunctions of this gene is well known for poor prognosis, but the mechanisms involved that lead from the gene defect to heart failure have not been clear. This has made it difficult to develop effective treatments.
The most striking finding from observing the gene-edited mice was that nuclear envelope ruptures triggered by the lack of Lamin A/C proteins began occurring before any other signs of heart disease, and ultimately involved surprisingly high numbers of muscle cells.
The nuclear envelope serves as a protective case around a cell’s DNA. If enough cells have such ruptures, it can lead to significant tissue damage and disrupt organ function.
Initially, the ruptures in the heart muscle cells were difficult to detect.
“We did not notice the ruptures at all for the first two years of investigation,” Ikegami says. “This is because the rupture occurs at a specific position on the surface of the nuclear envelope, and you cannot find the ruptures unless you see cells from the right angle.”
Findings challenge a prevailing theory
Until this study, many heart experts believed that nuclear envelope ruptures cause DCM by activating an immune response mechanism known as the cGAS-STING pathway.
However, that pathway was not activated in these mice, despite the high rates of nuclear envelope ruptures. Instead, the research team identified extracellular matrix (ECM) signaling from cardiomyocytes to fibroblasts as a key player in disrupting heart function.
These findings raise multiple implications that will require further research, Ikegami says.
Tests to detect nuclear envelope rupture potentially could serve as an early indicator of DCM, which might allow earlier intervention to head off heart failure. Preventing the ruptures, or finding ways to repair them, could slow or even prevent the progression of disease.
“We are interested in understanding why adult hearts are mainly affected in LMNA-DCM, even though the gene mutation is there from birth,” Ikegami says. “What are the special characteristics of child hearts that protect them from developing this disease during childhood?”
Meanwhile, nuclear envelope ruptures may be at the root of other “laminopathies” such as progeria (a rare premature aging disease linked to defective lamin A proteins) as well as some muscular dystrophies and lipodystrophies.
About the study
Co-authors on this study included Hanumakumar Bogireddi and Briana Thomas from Cincinnati Children’s and six researchers at the University of Chicago. The work also was supported by core facilities for animal care, microscopy, genomics, pathology, and viral vectors at Cincinnati Children’s.
Funding sources included the National Institutes of Health (R21/R33 AG054770, R01 HL163523, R01 HL124836, and R01 HL126509) and a Cincinnati Children’s Research Innovation and Pilot grant.
| Original title: | Pervasive nuclear envelope ruptures precede ECM signaling and disease onset without activating cGAS-STING in Lamin-cardiomyopathy mice |
| Published in: | Cell Reports |
| Publish date: | May 29, 2024 |
Research By

The Ikegami lab investigates mechanisms of gene regulation, chromosome organization, nuclear envelope functions, cardiovascular diseases, and age-associated diseases.