Potential Approach Emerges to Treat Autoimmunity and Prevent Serious Side Effects of Cancer Immune Therapies
Research By: Chandrashekhar Pasare, DVM, PhD
Post Date: January 21, 2022 | Publish Date: Jan. 21, 2022
Experts at Cincinnati Children’s report, in mice, that an antibody treatment can improve survival when certain type of ‘cytokine storm’ strikes
Whether it’s children coping with rare autoimmune diseases or cancer patients seeking out promising new immune therapies, more people are learning about an often-deadly form of immune system over-reaction called a “cytokine storm.”
Clinicians and scientists who have known about cytokine storms for a long time also know that many factors can be involved in triggering them, and only a few treatments can slow them down. Now, a team from Cincinnati Children’s reports early-stage success at taming some cytokine storms by disrupting signals emanating from activated T cells in our immune systems.
Detailed findings were published Jan. 21, 2022, in Science Immunology. The study has three lead authors: Margaret McDaniel, BS, Aakanksha Jain, and Amanpreet Singh Chawla, PhD, all formerly with Cincinnati Children’s. The senior corresponding author was Chandrashekhar Pasare, DVM, PhD, Professor, Division of Immunobiology and Co-Director of Center for Inflammation and Tolerance at Cincinnati Children’s.
“This discovery is important because we have shown, in mice, that the systemic inflammatory pathways involved in this type of T cell-driven cytokine storm can be mitigated,” Pasare says. “More work will be needed to confirm that the approach we used in mice can also be safe and effective for humans. But now we have a clear target to pursue.”
What is a cytokine storm?
Cytokines are tiny proteins secreted by virtually every type of cell. Dozens of known cytokines perform an array of vital, normal functions. In the immune system, cytokines help to guide T-cells and other immune cells to attack and clear away invading viruses and bacteria as well as combat cancer.
But sometimes, a cytokine “storm” results from having too many T cells in the battle. The result can be excess inflammation that can cause extreme, even fatal damage to healthy tissues.
The new research sheds light on the signaling process at the molecular level. The team reports that at least two independent pathways exist that trigger inflammation in the body. While there is a well-known and established path of inflammation for reacting to outside invaders, this work describes a less understood path that drives “sterile” or non-infection-related immune activity.
Hopeful news for cancer care
Two of the most exciting cancer care developments in recent years have been the development of checkpoint inhibitors and chimeric antigen receptor T cell therapy (CAR-T). These forms of treatment help T cells detect and destroy cancer cells that previously evaded the body’s natural defenses.
Several drugs based on CAR-T technology are approved for treating patents battling diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, mantle cell lymphoma, multiple myeloma, and B-cell acute lymphoblastic leukemia (ALL). Meanwhile. a number of checkpoint inhibitors are helping people with lung cancer, breast cancer and several other malignancies. These treatments include atezolizumab (Tecentriq), avelumab (Bavencio), cemiplimab (Libtayo), dostarlimab (Jemperli), durvalumab (Imfinzi), ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda).
However, for some patients, these treatments can allow swarms of rogue T-cells to attack healthy tissues as well as the cancer. In a series of mouse and laboratory experiments, the research team at Cincinnati Children’s reports tracking down the source of inflammation resulting from this T cell misbehavior and demonstrates a way to prevent it.
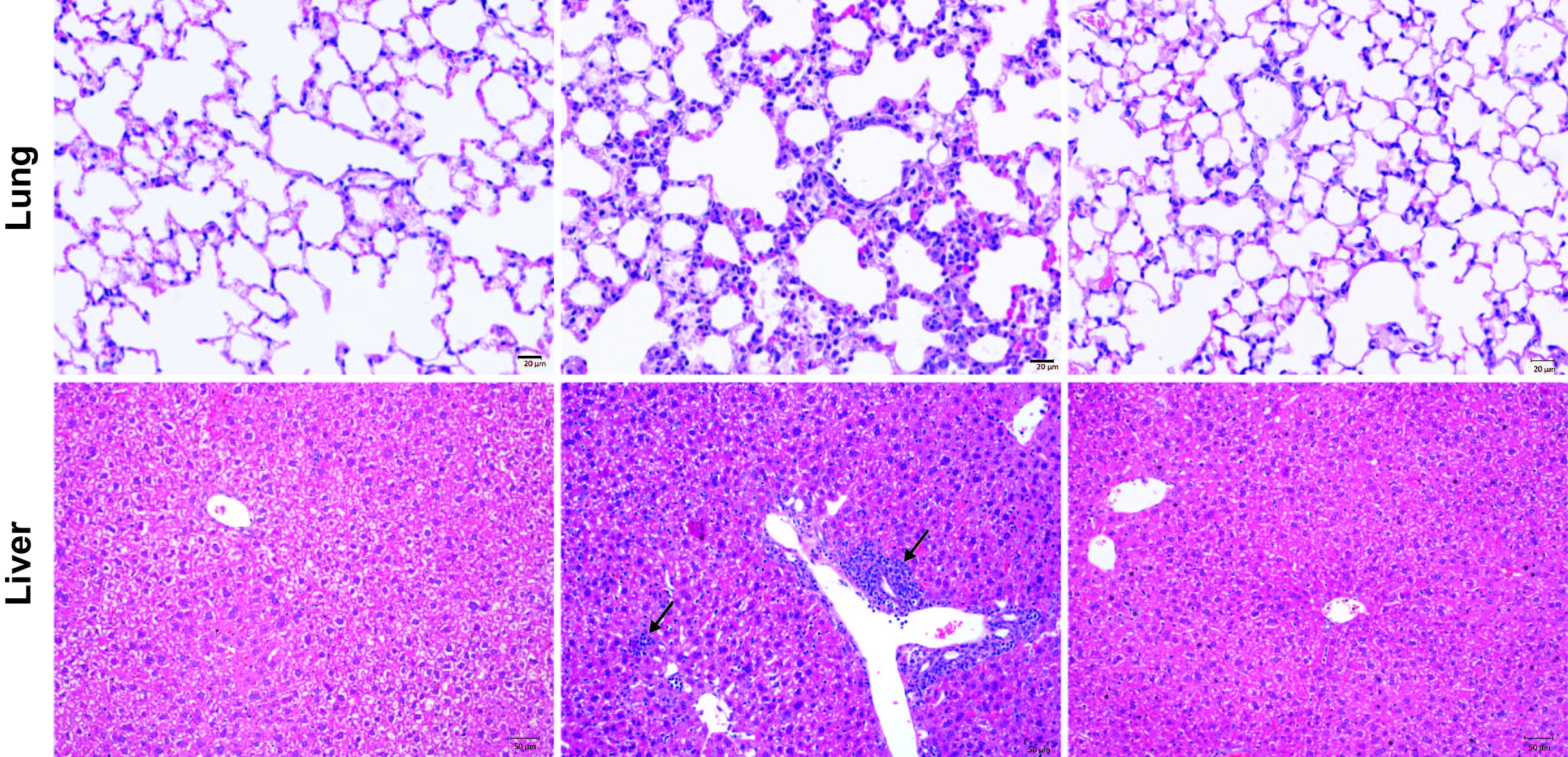
“We have identified a critical signaling node used by effector memory T cells (TEM) to mobilize a broad proinflammatory program in the innate immune system,” Pasare says. “We found that cytokine toxicity and autoimmune pathology could be completely rescued in multiple models of T cell-driven inflammation by disrupting these signals either through gene editing or with small molecule compounds.”
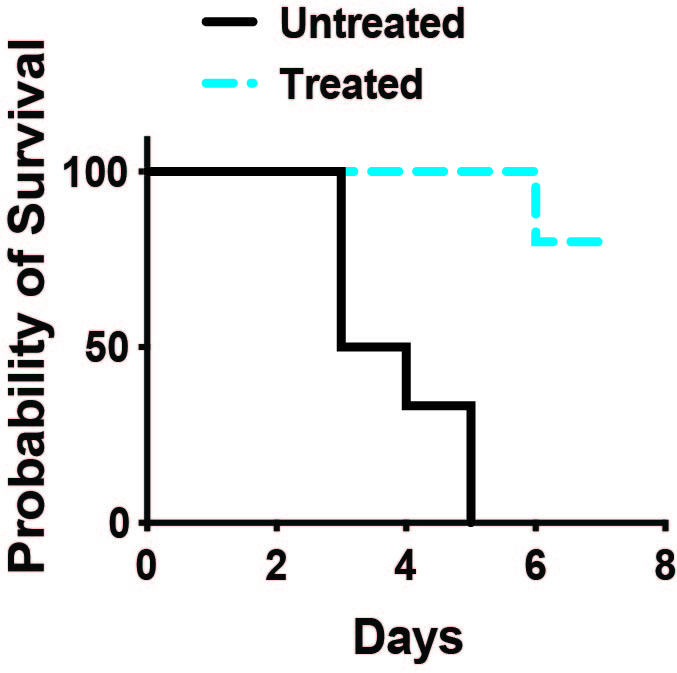
Without treatment, 100 percent of mice induced to experience a cytokine storm like those triggered by CAR-T therapy died within five days. But 80 percent of mice treated with antibodies to block signals emanating from activated T cells survived at least seven days.
Discovery not applicable to COVID-19
Many people with severe infections from the SARS-CoV-2 virus also have suffered cytokine storms. However, there are crucial differences between systemic inflammation triggered by a viral infection and this “sterile” form of runaway inflammation caused by activated T cells.
“We have identified a cluster of genes that are uniquely induced by TEM cells that are not involved in viral or bacterial infection response,” Pasare says. “This signifies a divergent evolution of these two mechanisms of innate activation.”
Next steps
In theory, an antibody treatment similar to that used in the mouse studies could be given to cancer patients before they receive CAR-T therapy. But more research is needed to determine if such an approach is safe enough to test in human clinical trials.
In addition to making a promising form of cancer care accessible to more people, controlling this sterile inflammation pathway might be helpful for children born with one of three very rare autoimmune diseases, including IPEX syndrome, which is caused by a mutation in the FOXP3 gene; CHAI disease, which results from malfunctions of the CTLA-4 gene; and LATIAE disease, caused by mutations in the LRBA gene.
About the study
Other Cincinnati Children’s co-authors for this study include: Hannah Meibers, BS, Irene Saha, PhD, Viral Jain, MD, Krishna Roskin, PhD, and Sing Sing Way, MD, PhD.
Funding sources for this study include the National Institutes of Health (AI123176 and AI155426) grants to Pasare and a National Science Foundation fellowship (2017220107) to McDaniel. Cincinnati Children’s has applied for a patent to cover methods for treating diseases by blocking TNF and CD40.
| Original title: | Effector memory CD4+ T cells induce damaging innate inflammation and autoimmune pathology by engaging CD40 and TNFR on myeloid cells |
| Published in: | Science Immunology |
| Publish date: | Jan. 21, 2022 |
Research By